New Drugs Approvals by FDA and EMA: 2020 Recap

The year 2020 was an eventful year for the pharmaceutical industry, with several companies across the world working at a feverish pace to find a treatment or a vaccine for the raging Covid-19, which has so far taken over 1.79 million lives worldwide.
With countries imposing lockdowns and regulators putting on-site inspections on hold, we were expecting far lower new drug approvals in mid-2020. But our mid-2020 recap published in July, which looked at new drug approvals by the US Food and Drug Administration (FDA) and European Medical Agency (EMA), found that the FDA had approved 33 new drugs by the end of June. This put the approvals within the ballpark of the past two years.
This week, we bring you a roundup of 2020, a tumultuous year when 58 drugs (53 approvals by the Center for Drug Evaluation and Research and 5 by the Center for Biologics Evaluation and Research) bagged FDA’s new drug approvals. While this number is lower than the number of drugs approved in 2018 (62), it is higher than the number for 2019 (54). Out of this, while 23 approvals were in the field of oncology, 9 were for infectious diseases and infections, 8 for genetic diseases, 7 for neurology, 3 for immunology and 2 for gastroenterology.
A year marked by EUA
With the pandemic raging across the world, emergency use authorizations (EUAs) dominated news headlines in 2020 — the FDA issued 10 EUAs, with the most prominent being those issued to Pfizer-BioNTech and Moderna for their Covid-19 vaccines.
EMA was busy as well since they issued 75 positive opinions with Novartis leading the pack with 8, followed by Pfizer and Sanofi which received 4 each. Click on the picture to access the free compilation by pharmacompass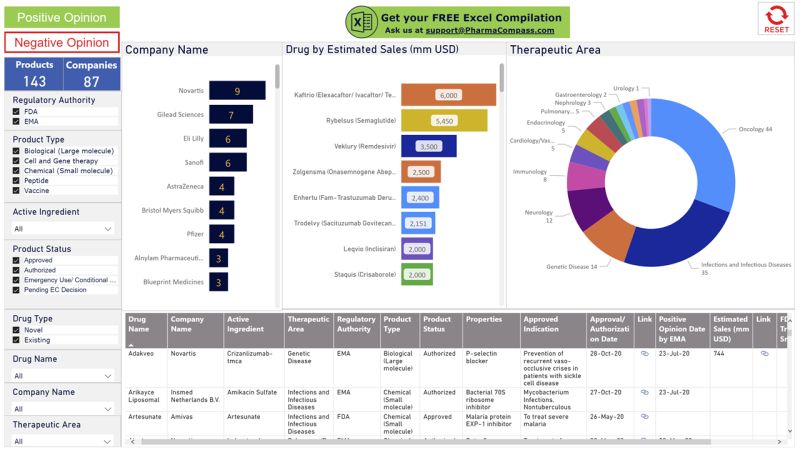 The EUAs came with their own set of controversies. In March, the FDA had issued an EUA “for oral formulations of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of” Covid-19. However, by June, FDA had revoked the EUA, as the agency determined that chloroquine and hydroxychloroquine were not likely to be effective in treating Covid-19 for the authorized uses in the EUA.
The EUAs came with their own set of controversies. In March, the FDA had issued an EUA “for oral formulations of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of” Covid-19. However, by June, FDA had revoked the EUA, as the agency determined that chloroquine and hydroxychloroquine were not likely to be effective in treating Covid-19 for the authorized uses in the EUA.
Amongst treatments for Covid-19, in May the FDA authorized the emergency use of Gilead’s antiviral drug remdesivir. In our mid-2020 recap, Gilead’s remdesivir was on top of our list of top-selling drugs after it received an EUA from the FDA.
In October, remdesivir became the first drug to be approved by the FDA for treatment of Covid-19 patients requiring hospitalization. While analysts predicted US$ 3.5 billion in revenue in early October, the future of this drug as a treatment for Covid-19 in hospitalized patients remains uncertain, especially in wake of results from the World Health Organization (WHO)-led Solidarity Trial that said Gilead’s remdesivir had little or no effect on the 28-day mortality or length of hospital stays for Covid-19 patients. The FDA approved remdesivir for hospitalized patients a week after the WHO results.
Gilead US$ 21 billion Immunomedics acquisition
Immunomedics’ antibody-drug conjugate (ADC) — Trodelvy (sacituzumab govitecan-hziy) — was approved by the FDA in April this year for the treatment of adult patients with metastatic triple-negative breast cancer who have received at least two prior therapies for the disease. Such tumor types account for 15 to 20 percent of breast cancers. Trodelvy follows remdesivir in our list of FDA approved drugs in 2020 with the highest sales potential. The current forecast for Trodelvy sales is US$ 2.151 billion by 2026. Continue reading on pharmacompass.com

