Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology
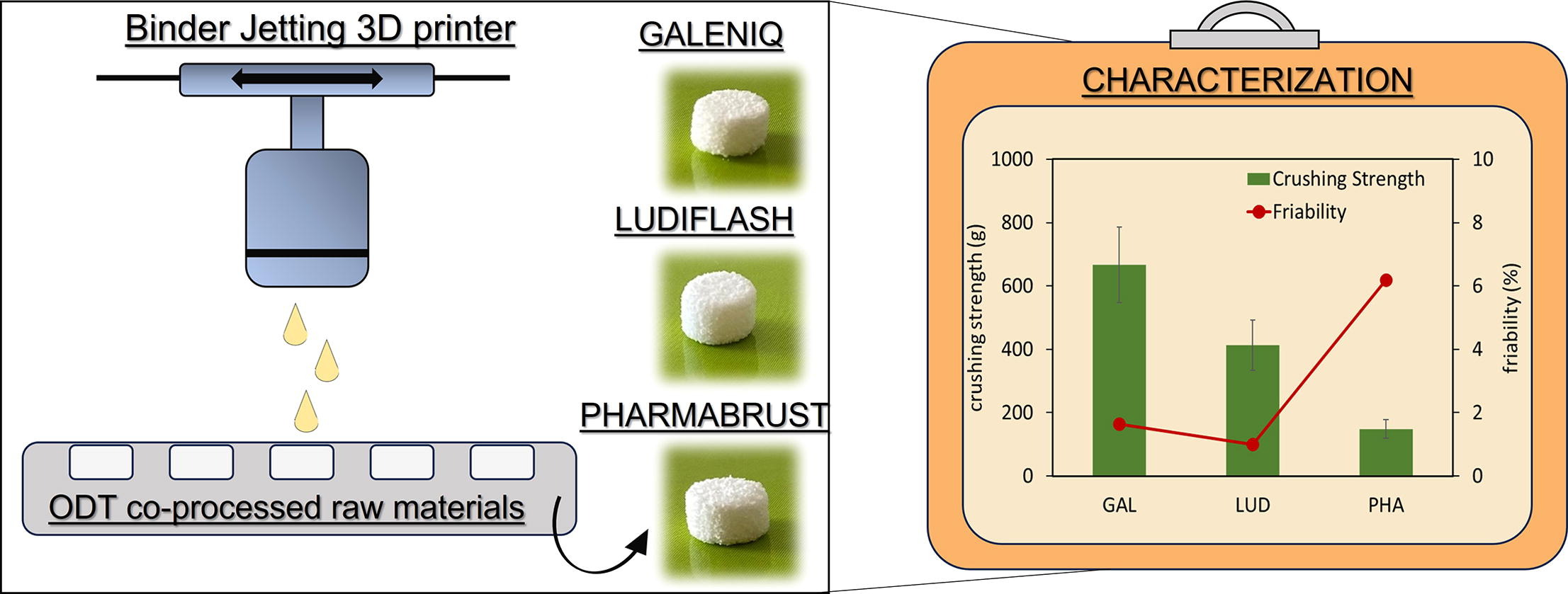
The use of co-processed materials for Orally Disintegrating Tablets (ODT) preparation by direct compression is well consolidated. However, the evaluation of their potential for ODT preparation by 3D printing technology remains almost unexplored. The present study aimed to estimate the use of commercially available co-processed excipients, conventionally applied in compression protocols, for the preparation of ODTs with binder jetting-3D printing technology. The latter was selected among the 3D printing techniques because the deposition of multiple powder layers allows for obtaining highly porous and easily disintegrating dosage forms.
The influence of some process parameters, including layer thickness, type of waveform and spread speed, on the physical and mechanical properties of the prototypes printed were evaluated. Our results suggested that binder jetting-3D printing technology could benefit from the co-processed excipients for the preparation of solid dosage forms. The process optimization conducted with the experiments reported in this work indicated that additional excipients were needed to improve the physical properties of the resulting ODTs.
Download the full article as PDF here Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology
or read it here
Materials
Powder blends were prepared with: GalenIQ™ 721 (GAL) (BENEO, D) or Ludiflash® (LUD) (Basf, D) or Pharmaburst® 500 (PHA) (SPI Pharma, USA), as ODTs co-processed excipients. Kollidon VA64 (KVA64) (BASF, D) was used as a polymer binder, then Aerosil® 200 (AER) (Evonik, D) (glidant) and mannitol (MAN) (Carlo Erba Reagents, I) (diluent) were also used.
GalenIQ™ 721 is a granulate of isomalt derived from beet sugar. Ludiflash® consists of four excipients: D-mannitol, crospovidone, polyvinyl acetate and povidone. Pharmaburst® 500 comprises mannitol, sorbitol, silicon dioxide and crospovidone. All co-processed are well known and are specific for the preparation of ODTs by direct compression [28].
For the liquid binder: povidone (Kollidon 30) (K30) (binder polymer), sodium lauryl sulfate (Kolliphor® SLS Fine) (SLS) (BASF, D) (surfactant), ethanol (ETH), glycerine (GLY) (humectant), propylene glycol (PPG) (Carlo Erba Reagents, I) (humectant) and deionized water (DEW) (Millipore, USA) were used.
Evelyn Ochoa, Lucia Morelli, Lucia Salvioni, Marco Giustra, Beatrice De Santes, Francesca Spena, Linda Barbieri, Stefania Garbujo, Matteo Viganò, Brian Novati, Giulia Tomaino, Saliha Moutaharrik, Davide Prosperi, Luca Palugan, Miriam Colombo, Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology, European Journal of Pharmaceutics and Biopharmaceutics, 2023, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.11.023.
Read more on Overview of Pharmaceutical 3D printing here:


