Oral administration of zein-based nanoparticles reduces glycemia and improves glucose tolerance in rats
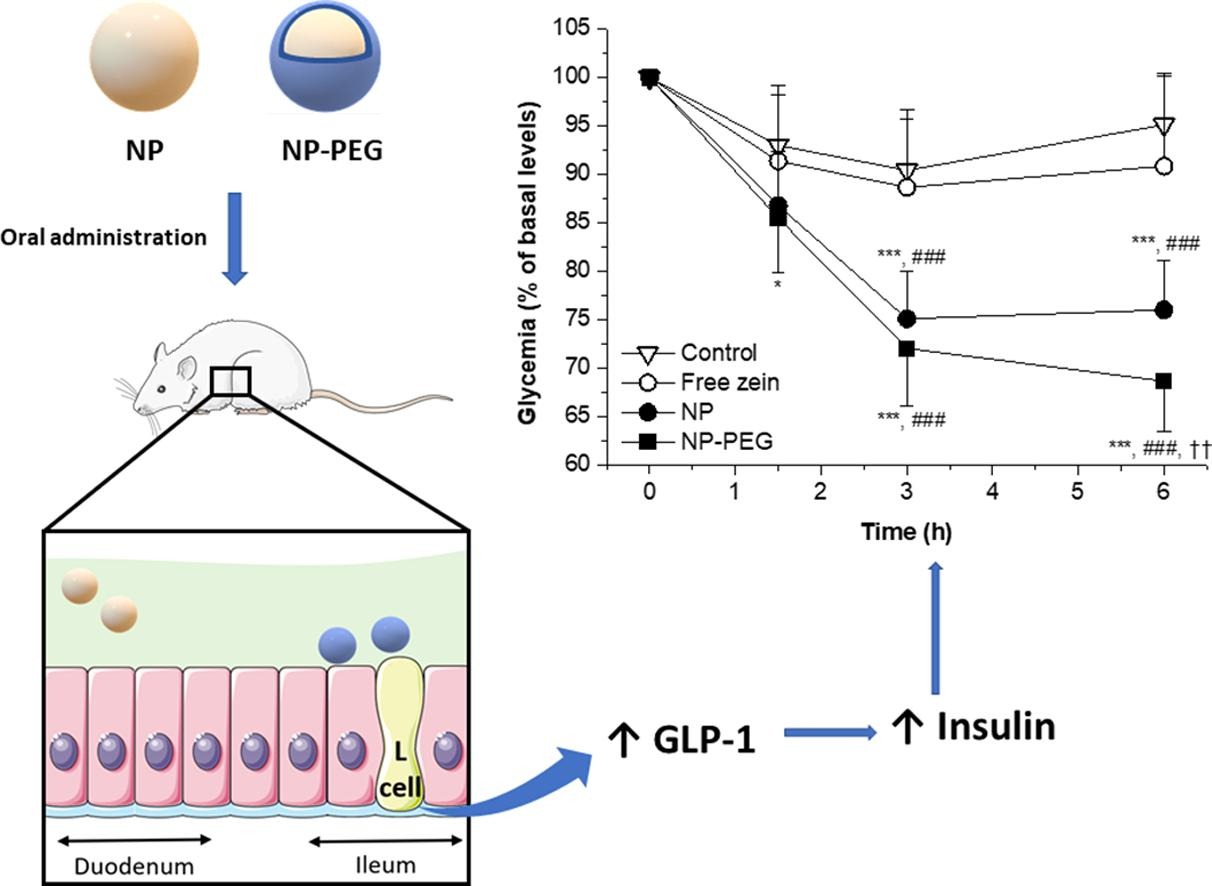
The aim was to evaluate the effect of zein-based nanoparticles on the glucose homeostasis, following oral administration to Wistar rats. For this purpose, bare nanoparticles (NP, with tropism for the upper intestinal regions) and poly(ethylene glycol)-coated nanoparticles (NP-PEG), with the capability to reach the ileum and cecum of animals, were evaluated. Both formulations were spherical in shape, displaying sizes around 200 nm and a negative surface zeta potential. The oral administration of a single dose of these nanoparticles to animals (50 mg/kg) induced a significant decrease of the glycemia, compared control rats and in animals treated with the free protein (p < 0.001). Moreover, these nanoparticles improved the glycemic control against an intraperitoneal glucose tolerance test; particularly NP-PEG.
Highlights
• Zein nanoparticles, orally administered, induce hypoglycemic response in healthy rats.
• PEG-coated nanoparticles are more effective in decreasing glycemia than bare ones.
• The hypoglycemic effect of zein nanoparticles would be mediated by GLP-1 secretion.
• Increased GLP-1 levels in blood would improve glycemic control against an ipGTT.
These findings would be due to an increased release of glucagon-like peptide-1 (GLP-1) by l-cells, which are more abundant in distal regions of the intestine. In fact, the GLP-1 blood levels of animals treated with nanoparticles were significantly higher than controls (about 40 % and 60 % for NP and NP-PEG groups, respectively). This higher capability of NP-PEG, with respect to NP, to increase the release of GLP-1 and control glycemia would be related to its ability to reach the distal areas of the small intestine.
1. Introduction
Glucose homeostasis is a complex phenomenon involving a wide variety of hormones whose objective is to keep the blood glucose levels within the normal range (4 – 6 mM), avoiding the conditions of hyper- and hypoglycemia (Röder et al., 2016). Insulin is the principal hypoglycemic hormone, and it is produced and stored in the β cells of the pancreas. These cells that act as glycemic sensors are found forming clusters, the so-called islets of Langerhans (Fu et al., 2012). When the glycemia rises, glucose enters the β cells through the GLUT transporters and triggers a signaling cascade that induces an increase in the intracellular calcium concentration and, in last term, leads to the exocytosis of the insulin vesicles (Henquin et al., 2006, Röder et al., 2016). Through the bloodstream, insulin reaches sensitive tissues (e.g., adipose tissue, muscle and liver) and stimulates glucose uptake by the cells, decreasing the circulating levels of this saccharide (Cheng et al., 2010, Jung and Choi, 2014, Lázár et al., 2018). Although glucose is the main inductor for insulin release, other types of nutrients (i.e., amino acids and di- tripeptides) can also act as insulinogogues (Deeney et al., 2000, Fu et al., 2012). However, the mechanisms by which amino acids induce secretion of insulin are very variable, depending on their nature and physicochemical properties (McClenaghan et al., 1996).
After food ingestion, the rise in the blood insulin levels has been shown to be more potent than during an isoglycemic intravenous glucose infusion. This phenomenon is known as the incretin effect and it happens because the presence of food in the lumen stimulates the production of hormones by the enteroendocrine cells present in the gut (Kazafeos, 2011, Mortensen et al., 2003). The hormones involved in this response are the incretins GLP-1 and GIP, from which GLP-1 appears to play a more important role in glucose homeostasis (Holst, 2019).
GLP-1 is stored in granules within the L cells of the gut, whose abundancy increases from proximal to distal regions of the intestine (Pais et al., 2016a). These cells act as sensors and, in response to the presence of nutrients in the lumen, release their GLP-1-containing granules (Müller et al., 2019). The release of GLP-1 starts a few minutes after the ingestion of the meal and lasts for several hours (Pais et al., 2016a). Carbohydrates, fats, and proteins present in the lumen act as GLP-1 release inductors. Regarding the secretagogue effect of proteins and their metabolites, several peptones and amino-acids (e.g. glutamine, glycine, alanine, phenylalanine and arginine) have shown the capability of directly induce GLP-1 release (Pais et al., 2016b), although oligo- or large peptides seem to be more potent than free amino acids (Ishikawa et al., 2015). Independently of the insulinogogue effect of GLP-1, which is always glucose-dependent (Clemmensen et al., 2013), it also induces glucose uptake by muscle, adipose tissue and liver (Müller et al., 2019, Rowlands et al., 2018). Moreover, in the liver, GLP-1 also induces hepatic glucose clearance and glycogen synthesis while reducing the hepatic glucose production through the inhibition of glucagon release (Jin and Weng, 2016).
On the other hand, GIP is produced and secreted by the enteroendocrine K cells localized in the gastrointestinal tract. In contrast to the distribution of L cells, K cells are mainly located in proximal regions of the gut, decreasing in number in further sections (Pais et al., 2016a). Although K cells are sensitive to all type of nutrients (sugars, fats and proteins), it has been observed that proteins are the most potent inductors of GIP release (Seino et al., 2010). GIP exerts its function mainly over the pancreas, the bone, the adipose tissue, and the brain. However, in contrast to the effect exerted by GLP-1 in the pancreas, GIP can stimulate both the production of insulin and glucagon (Seino et al., 2010). When glycemia is low (around 4 mM, as in fasted state), GIP induces glucagon release by targeting α cells. However, when blood glucose levels rise (postprandial state), it stimulates insulin release by targeting the β cells (Kimberley and Campbell, 2020).
Zein is an alcohol-soluble protein, with a GRAS regulatory status, that has been extensively proposed as raw material for the preparation of nanoparticles for the oral delivery of biologically active compounds (Luo and Wang, 2014, Reboredo et al., 2022, Weissmueller et al., 2016). This popularity would be related with a high loading capability for lipophilic compounds, as well as with an important facility to decorate the surface with hydrophilic compounds and, thus, modify their biodistribution in vivo (Martínez-López et al., 2020). On the other hand, zein would induce some beneficial biological effects related to glucose homeostasis. Thus, it has been shown that the ileal administration of zein hydrolysates would decrease glycemia levels in rats, improving the glucose tolerance, by stimulating the secretion of GLP-1 (Higuchi et al., 2013, Mochida et al., 2010). Based on these previous reports, orally administered zein-based nanoparticles could, potentially, induce the secretion of incretins and, hence, improve the glucose homeostasis. If so, these kinds of nanoparticles could be used alone or in combination with other hypoglycemic drugs to improve the glucose management in diabetic patients.
In this context, the aim of this work was to evaluate the in vivo effect of two different types of zein nanoparticles on the glucose homeostasis, following oral administration. For this purpose, bare nanoparticles (NP), which possess mucoadhesive properties (Inchaurraga et al., 2019), and poly(ethylene glycol)-coated nanoparticles (NP-PEG), with a mucus-diffusive demeanor (Reboredo et al., 2021), were evaluated in healthy Wistar rats.
Download the full article as PDF here Oral administration of zein-based nanoparticles reduces glycemia and improves glucose tolerance in rats
or read it here
Cristian Reboredo, Carlos J. González-Navarro, Ana L. Martínez-López, Juan M. Irache, Oral administration of zein-based nanoparticles reduces glycemia and improves glucose tolerance in rats, International Journal of Pharmaceutics,
Volume 628, 2022, 122255, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2022.122255

