Preparation, optimization and evaluation of Osthole transdermal therapeutic system
In the current study, the solubility and permeability of Osthole-loaded microemulsion were enhanced, which increased bioavailability. In addition, Carbomer 940 was added for prolonged drug delivery. The microemulsion was prepared after the screening of Kukui oil, Labrasol (surfactant), and transcutol-P (co-surfactant). Pseudoternary phase diagrams were employed to find the microemulsion region. Box Behnken Design (BBD) was employed for optimizing microemulsions. Variables were related and compared using mathematical equations and response surface plots (RSP). MEBG was then compared with control gel on the basis of stability studies, drug permeation, skin irritation studies, and anti-inflammatory studies. Microemulsion preparations depicted a pH of 5.27 – 5.80, a conductivity of 139 – 185 μS/cm, a poly-dispersity index of 0.116 – 0.388, a refractive index of 1.330 – 1.427, an average droplet size of 64 – 89 nm, homogeneity, spherical shape, viscosity 52 – 185 cP. Predicted values of Optimized microemulsions showed more reasonable agreement than experimental values. The microemulsion was stable and non-irritating on Rabbit skin. MEBG showed a significant difference from control gel for percent edema inhibition from the standard. The permeation enhancing capability of MEBG using a suitable viscosity fabricates it promising carrier for transdermal delivery of Osthole.
Introduction
The substance Osthole is extracted from annual herb fruit in the family Umbelliferae, and it has a yearly herb yellow green to white crystalline powder appearance. It belongs to the coumarin compound. Its chemical name is 7-methoxy-8-isopentenyl coumarin, its molecular weight is 244.29 and its molecular formula is C15H16O3. It depicted antispasmodic, anti-arrhythmic, antihypertensive, antitumor effects, anti-inflammatory and immune-enhancing functions (You et al. 2009, Liao et al. 2010).
The microemulsion is a single, optically isotropic, thermodynamically stable liquid solution, with a droplet between 10 and 100 nm. It comprises the oil phase, a surfactant, a cosurfactant and an aqueous phase that is transparent, translucent and thermodynamically stable. The microemulsion merits include enhanced solubility and bioavailability by increasing its shelf life. The benefits of transdermal administration of microemulsion comprise an enhancement in drug concentration upon the skin that increases drug concentration in preparation. Secondly, an improved drug thermodynamic activity may enable its skin distribution, which is helpful for transdermal absorption. Moreover, microemulsion components can facilitate penetration enhancers by reducing the stratum corneum (SC) diffusion barrier and enhancing the rate of drug penetration across the skin (Zhu et al. 2009, Lawrence & Rees 2000).
Transdermal delivery is a potential route of administration. Ointments, Gels, etc., cannot permeate across the skin due to the skin barrier nature of the stratum corneum. However, subcutaneous delivery is painful for penetration to skin depths. The microemulsion is considered a new drug carrier system that can effectively permeate the skin for drug delivery (Chen et al. 2007).
Microemulsions are applied transdermally to enhance the drug’s bioavailability and minimize the occurrence of adverse reactions compared to oral products. Despite that, high fluidity and microemulsions limit the release and the absorption of drug. Consequently, MEBG was formulated to increase the absorption and bioavailability of the drug (Chen et al. 2006).
Response Surface Methodology is the optimization of independent variables for estimating dependent variables using BBD for creating polynomial equations by 1st, 2nd and quadratic models. It demands little time and experimentation than needed for manufacturing conventional dosage forms. It comprises Q24, flux and lag time-dependent variables, and oil, Smix, and water) independent variables (Box & Behnken 1960, Gannu et al. 2010).
The current study successfully prepares a new oil/water gel base containing microemulsion for Osthole transdermal delivery to enhance systemic bioavailability by increasing solubility, reducing oral gastric toxicity, and improving permeability. First, BBD was employed for optimizing independent variables via estimating dependent variables. Then, atomic force microscopy (AFM) forms were characterized, pH, zeta potential and size, viscosity, skin irritation, conductivity, refractive index and stability. Further, optimized formulations were compared for in vitro release/permeation and anti-inflammatory studies from control.
Materials and Methods
Experimental
Osthole was purchased from YuanYe Biological Technology Co. Ltd. (Shanghai, China), Kukui oil, soybean oil, sesame oil, sunflower oil, eucalyptus oil, oleic acid, isopropyl myristate, castor oil, almond oil, olive oil, nutmeg oil, tween 20, tween 80, isopropanol, ethanol, propylene glycol were obtained from Merck, Germany.
Construction of Pseudoternary phase diagram for microemulsions
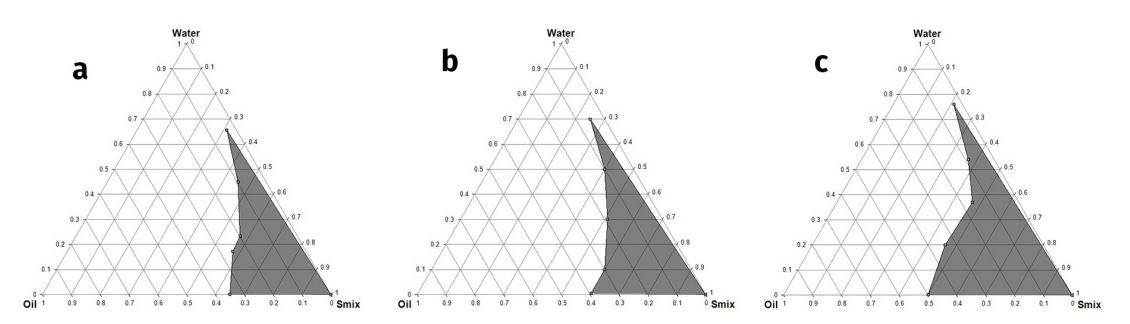
Selected microemulsion components Kukui oil, Labrasol, Transcutol-P and water after solubility studies were used for constructing Pseudoternary phase diagrams using the water titration method for getting components concentration ranges for preparing microemulsons. Surfactant to co-surfactant weight ratios was assorted as 1:1, 2:1, and 3:1. Fixed surfactant to co-surfactant (Smix) weight ratio was mixed with oil at a ratio of 1:9, 1:8, 1:7, 1:6, 1:5, 1:4, 1:3.5, 1:3, 1:2.33, 1:2, 1:1.5, 1:1, 1:0.67, 1:0.43, 1:0.2 and 1:0.11 for each pseudoternary phase diagram (Sahoo et al. 2014). Water was added drop-wise into each mixture of oil and Smix under magnetic stirring at ambient temperature. As a result, the mixture became clear and transparent. Pseudoternary phase diagrams were constructed to find out the optimum ranges of microemulsion components Figure 1(a,b,c).
Download the full study as PDF here: Preparation, optimization and evaluation of Osthole transdermal therapeutic system
or read it here
Muhammad Naeem, Taniya Iqbal, Muhammad Yousuf, Zarqa Nawaz, Sajjad Hussain, Abdulhakeem S. Alamri, Charis M. Galanakis, Atif Ali, Preparation, optimization and evaluation of Osthole transdermal therapeutic system, Biomedical Sciences, An. Acad. Bras. Ciênc. (2023) 95 (3), Online ISSN 1678-2690
https://doi.org/10.1590/0001-3765202320221023

