Combining the potential of 3D printed buccal films and nanostructured lipid carriers for personalised cannabidiol delivery
Abstract
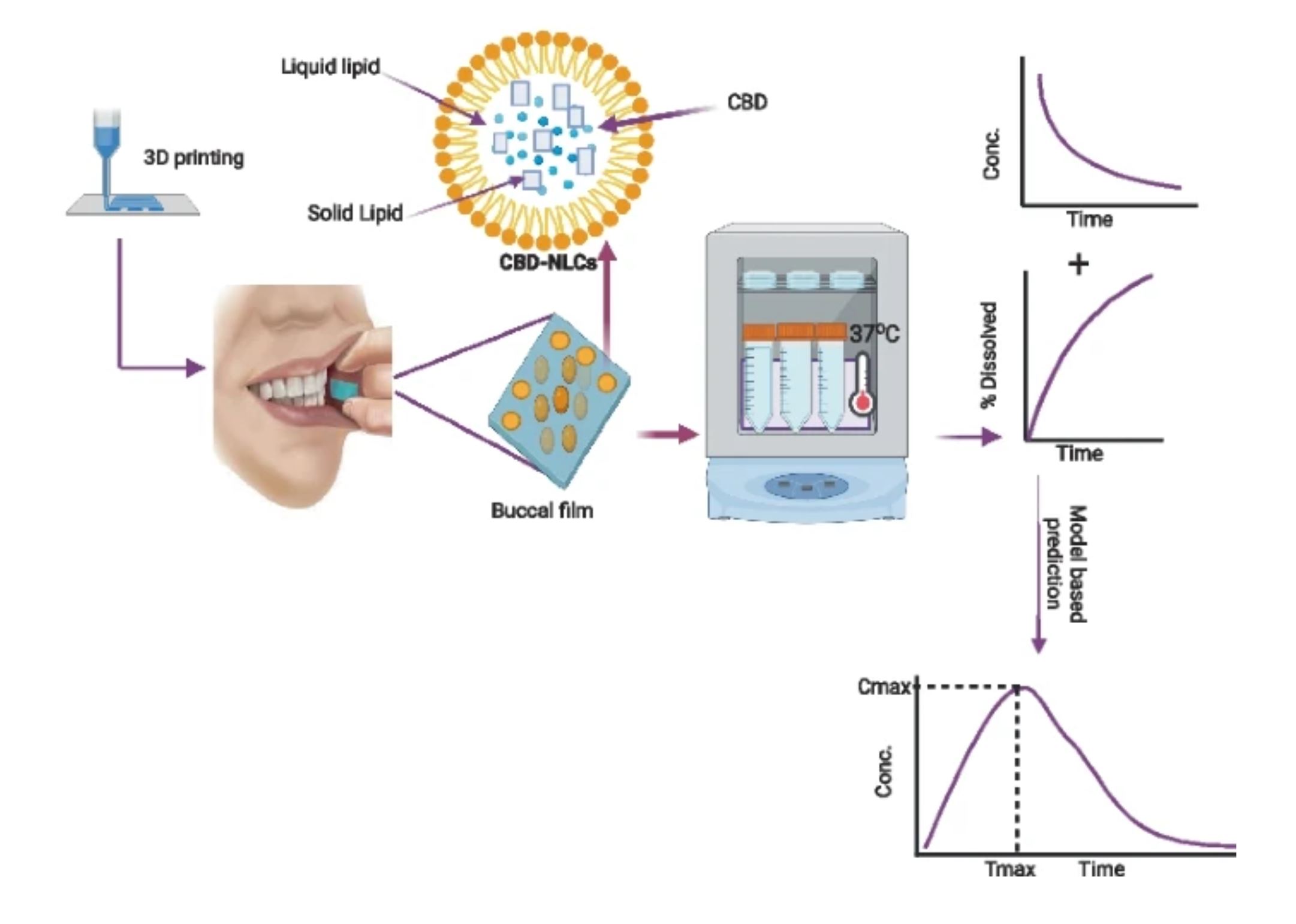
Cannabidiol (CBD) has been recognized for its numerous therapeutic benefits, such as neuroprotection, anti-inflammatory effects, and cardioprotection. However, CBD has some limitations, including unpredictable pharmacokinetics and low oral bioavailability. To overcome the challenges associated with CBD delivery, we employed Design of Experiments (DoE), lipid carriers, and 3D printing techniques to optimize and develop buccal film loaded with CBD-NLCs. Three-factor Box-Behnken Design was carried out to optimise the NLCs and analyse the effect of independent factors on dependent factors. The emulsification-ultrasonication technique was used to prepare the NLCs. A pressure-assisted micro-syringe printing technique was used to produce the films. The produced films were studied for physicochemical, and mechanical properties, release profiles, and predicted in vivo performance. The observed particle size of the NLCs ranged from 12.17 to 84.91 nm whereas the PDI varied from 0.099 to 0.298. Lipid and sonication time positively affected the particle size whereas the surfactant concentration was inversely related. CBD was incorporated into the optimal formulation and the observed particle size, PDI, and zeta potential for the CBD-NLCs were 94.2 ± 0.47 nm, 0.11 ± 0.01 and − 11.8 ± 0.52 mV. Hydroxyethyl cellulose (HEC)-based gel containing the CBD-NLCs was prepared and used as a feed for 3D printing. The CBD-NLCs film demonstrated a slow and sustained in vitro release profile (84. 11 ± 7.02% in 6 h). The predicted AUC0–10 h, Cmax, and Tmax were 201.5 µg·h/L, 0.74 µg/L, and 1.28 h for a film with 0.4 mg of CBD, respectively. The finding demonstrates that a buccal film of CBD-NLCs can be fabricated using 3D printing.
Introduction
Cannabidiol (CBD) is a non-psychoactive phytocannabinoid with several reported pharmacological effects including neuroprotection, cardioprotection, and anti-inflammatory effects [1, 2]. CBD has low toxicity, non-hallucinogenic effects, and is well tolerated at high doses, compared to other cannabinoids [3, 4]. Epidiolex®, the only marketed CBD monotherapy, has been approved by the European Medicines Agency (EMA) and FDA for tuberous sclerosis complex, Dravet syndrome, and Lennox-Gastaut syndrome associated seizures [5]. Additionally, a buccal spray called Sativex® containing a 1:1 ratio of CBD and delta-9 tetrahydrocannabinol (THC) has been approved in over 25 countries for the treatment of muscle spasms related to multiple sclerosis [6].
Despite its potential advantages, CBD has unpredictable pharmacokinetics and low oral bioavailability (6%) mainly due to its significant presystemic metabolism, high lipophilicity (log P = 6.3), and low water solubility [7, 8]. Furthermore, CBD is unstable in gastric pH, highlighting the need to consider optional routes and drug delivery systems [9]. Several cannabinoids, including CBD, start to degrade at a temperature as high as 160 °C, resulting in decreased quantities [10].
Buccal drug delivery offers great advantages over other routes including oral and parenteral administrations [11]. It is a non-invasive, painless, and convenient method of drug administration [12]. Furthermore, this route bypasses both the enzymatic degradation in GI tract tract and hepatic first-pass metabolism, making it an ideal delivery route for drugs that undergo enzymatic degradation such as CBD [13]. It also allows direct systemic delivery of drugs due to the rich blood supply to the region. It is important to note that a buccal administration route is a viable option for patients who have difficulty swallowing, leading to improved treatment outcomes and better patient experiences. Buccal films are considered a patient-friendly dosage form due to their small size, ease of use, and storage. They can also be administered with minimal water, making them an ideal delivery system for many drugs [14]. Buccal films can also have multiple layers, allowing for sustained drug release within the oral cavity [15].
The utilization of nanoparticles based on lipids has been proposed as a compelling strategy to improve the solubility and bioavailability of drugs that have low water solubility, regulate release kinetics, and increase drug loading capabilities [16, 17]. They can be administered by a variety of routes including parenteral, mucosal, dermal, pulmonary, and topical [18,19,20]. NLCs are a newer type of lipid nanoparticle that contains a mixture of liquid and solid lipids, plus a surfactant at room temperature [21]. NLCs have many benefits over traditional carriers, including improved bioavailability and permeability, lower risk of side effects, and the ability to be produced on a large scale. In comparison to Solid Lipid Nanoparticles (SLNs), NLCs offer a greater drug-loading capacity for certain drugs and minimal drug expulsion during storage [22, 23].
3D printing, on the other hand, has gained significant attention as a progressive innovation in the pharmaceutical field and is expected to revolutionalize drug manufacturing [24]. Its use has expanded exponentially in recent years due to its potential advantages, including producing a personalized dose form with a specific shape, modified release kinetics, and color thereby ensuring patient-centricity [25,26,27]. Furthermore, 3D printing is able to produce a high-quality product, within minutes, saving time and resources [25].
3D printers produce dosage forms from digital models by gradually depositing material at precise locations in a layer-by-layer fashion [28,29,30]. The 3D printers commonly used in the pharmaceutical field are stereolithography (SLA) [31], inkjet, semi-solid extrusion, fused deposition modelling (FDM), binder-jetting, and selective laser sintering (SLS) printing [8, 9]. In semi-solid extrusion, objects are created by step-by-step deposition of layers of feed material, often paste or gel [10]. It offers several advantages, including the ability to print at low temperatures, fast printing speed, and meeting quality requirements [11].
The number of scientific articles on 3D printing for drug delivery has significantly increased over the last 10 years confirming the growing interest in the use of 3D printers for drug development [32]. Of note, the feasibility of 3D printing to produce tailored pharmaceutical dosage forms has also been proven by the FDA’s approval Spritam® (levetiracetam) in 2015, 3D printed orodispersible tablet [33,34,35].
The advantages of 3D printing in developing personalised pharmaceutical formulations have been widely recognized [36, 37]. Customized dosage forms can be quickly produced by modifying their design using a computer-aided design (CAD) file. The customization considers individual patient needs including age, weight, organ function, disease condition, and patient preferences. Multiple drugs can also be printed in a single dosage form addressing the issue of polypharmacy and related medication adherence issues [38, 39].
Considering the challenges associated with oral CBD administration and the growing need to personalize therapy, we developed a buccal film of CBD using semi-solid extrusion 3D printing technology. Combining the advantages of buccal films, lipid-based nanoparticles, and 3D printing into a single system would improve the delivery of CBD. In addition, the NLC formulation was optimized using the Box-Behnken design. This design is a type of Response Surface Methodology (RSM) that is commonly used to optimise formulations as it requires fewer runs and less time compared to other methods [20]. RSM involves the application of mathematical and statistical techniques to analyse formulation obstacles and process parameters, facilitating the analysis and modeling of the relationship between the obtained response surfaces and the controllable input parameters [40, 41].
Buccal films containing SLNs of drugs were previously shown to improve the solubility and bioavailability of drugs [42, 43]. As far as our knowledge is concerned, this is the first study that reported NLCs-loaded buccal films. In this study, we developed a CBD buccal drug delivery system containing NLCs of CBD. The formulation could potentially improve the low bioavailability and variable pharmacokinetics of CBD. Polymers with mucoadhesive property were used to increase the bio-adhesiveness of the film. The film was characterized for physicochemical properties, mechanical properties as well as in vitro release properties. In vivo performance of the drug was predicted using a convolution method in R programming language.
Download the full article as PDF here Combining the potential of 3D printed buccal films and nanostructured lipid carriers for personalised cannabidiol delivery
or read it here
Materials
CBD was sourced from PM Separations in Queensland, Australia, and had a purity of ≥ 98%. Glyceryl distearate (Precirol® ATO 5) was obtained from Gattefosse in Lyon, France. Hydroxyethyl cellulose NF was provided by Medisca (NY, USA). Sigma-Aldrich in New South Wales, Australia provided polyethylene glycol 400, Tween 80®, and liquid oil capric/caprylic triglycerides. Deionised water with a resistivity of 18.2 MΩ at 25 °C was used to prepare the formulations and all chemicals were of the highest commercial grade available.
Abdella, S., Kim, S., Afinjuomo, F. et al. Combining the potential of 3D printed buccal films and nanostructured lipid carriers for personalised cannabidiol delivery. Drug Deliv. and Transl. Res. (2023). https://doi.org/10.1007/s13346-023-01446-0
Read more on The Role of Excipients in CBD Products here:


