The processes behind drug loading and release in porous drug delivery systems
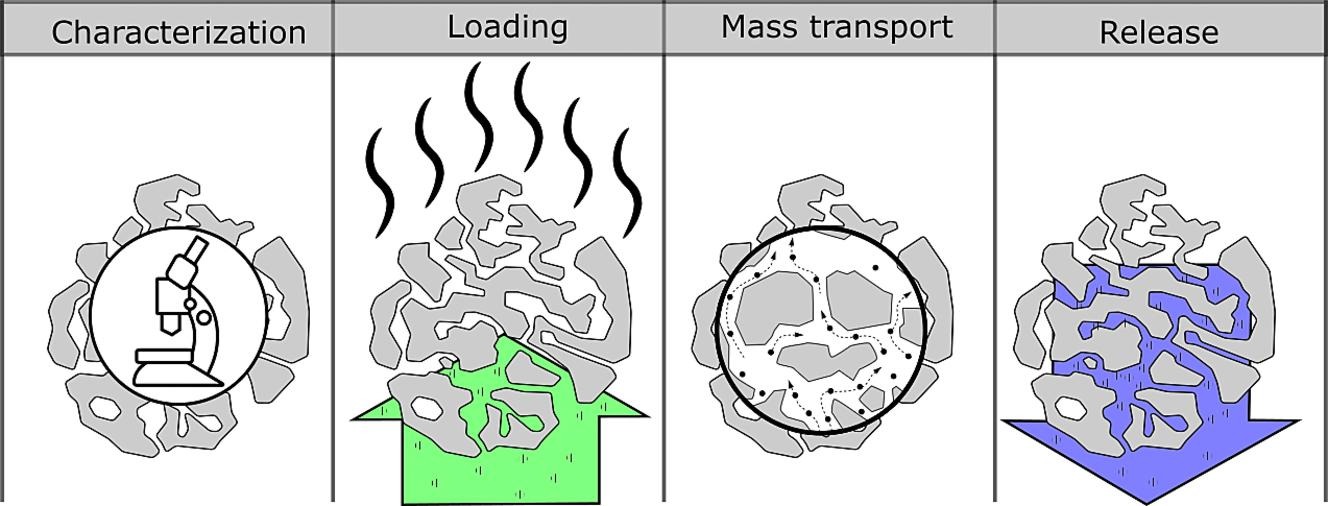
Porous materials are ubiquitous and exhibit properties suitable for depositing therapeutic compounds. Drug loading in porous materials can protect the drug, control its release rate, and improve its solubility. However, to achieve such outcomes from porous delivery systems, effective incorporation of the drug in the internal porosity of the carrier must be guaranteed. Mechanistic knowledge of the factors influencing drug loading and release from porous carriers allows rational design of formulations by selecting a suitable carrier for each application. Much of this knowledge exists in research areas other than drug delivery. Thus, a comprehensive overview of this topic from the drug delivery aspect is warranted. This review aims to identify the loading processes and carrier characteristics influencing the drug delivery outcome with porous materials. Additionally, the kinetics of drug release from porous materials are elucidated, and the common approaches to mathematical modeling of these processes are outlined.
Introduction
Porous materials exhibit an enlarged surface area and large pore volumes that allow the adsorption and deposition of active compounds. These key characteristics make porous materials attractive for drug delivery purposes. Drug loading inside the porous structures is done to achieve control over the drug release rate [1], [2], [3], [4], [5], producing floating gastro-retentive dosage forms[6], [7] and protecting the drug from detrimental external conditions[8], [9], [10], [11], [12]. Additionally, porous materials have been frequently used to modify the dissolution rate of low-soluble drugs [13], [14], [15], [16], [17], [18]. The large surface area of these materials can increase the rate of dissolution of the compounds distributed on their surface[19], [20], [21]. The large surface area also contributes to the amorphization of the drug, and the confined spaces of the pores can prevent the crystallization of the drug[22], [23], [24], [25], [26]. Amorphous materials have a higher energy state and dissolve more readily than crystalline solids. Apart from the drug delivery purpose, the potential of the porous materials to adsorb and accumulate external payload in their internal structure has been the basis of employing these materials for other applications such as drying of gases[27], synthesis of supported catalysts[28], [29] as well as processes that rely on the adsorption phenomena such as separation processes[30], [31], [32]. The porous structure has been used in the biomedical area for cell infiltration and tissue growth, e.g., bone and cartilage regeneration devices and drug delivery implants [33], [34].
The International Union of Pure and Applied Chemistry (IUPAC) classifies pore sizes into three main categories: micropores < 2 nm, mesopores 2–50 nm, and macropores > 50 nm[35]. Based on these definitions, the porous materials with the latter pore sizes are called microporous, mesoporous, and macroporous, respectively. The term nanoporosity has also been used without a precise definition. Still, a nanoscale range of about 0.1–100 nm can be assumed as the likely pore size range for this term. The latter means nanopores could fall under any IUPAC pore size definitions[36].
Individual pores can be classified based on their geometry and location within a porous system: Pores accessible to the surrounding medium are called open pores (Fig. 1a-d). These pores can be opened only from one end and are described as blind pores (Fig. 1b,d,g,(e)). Those pores accessible from two or more ends are called the through pores (Fig. 1a and c). The third type of pores in porous media are closed pores. These pores do not allow mass exchange with the surroundings (Fig. 1e and f). That can be due to the complete isolation of the pore within the skeletal structure of the particle (Fig. 1f), or the pore opening might be smaller than the molecular size of a substance (Fig. 1e). Ruike et al. [37] define a closed pore as a pore that helium cannot penetrate at 303 K. From a practical point of view, a closed pore can also be defined based on accessibility to a substance of interest. Furthermore, pores can be divided based on geometrical characteristics. For this purpose, we propose a two-level hierarchical classification approach. The first level describes the ratio between a pore’s body and its throat (b/t-ratio). That includes three basic geometrical shapes: Cylindrical pores have a constant diameter throughout their length and, therefore, a b/t-ratio of 1 (Fig. 1b, c, g). Pores with body diameters smaller than their throat have a cone or funnel shape. In these pores, the diameter converges, and therefore, the b/t-ratio is < 1 (Fig. 1, type a). If the pore throat is smaller in diameter than the pore’s body, this shape is called an ink-bottle pore. The pore diameter is different throughout its length, leading to a b/t-ratio > 1 (Fig. 1d). The second level of our suggested classification system describes the geometry of the pores’ cross-section can be circular, oval, square, rectangular, or hexagonal, among others.
Porosity is defined as the ratio of the void volume over the total volume of material, and it is often expressed as a fraction of unity or in percentages.
In the pharmaceutical area, porous microparticulate materials are often used as a delivery device and to prepare dosage forms such as compacted or sintered products. Therefore, it is essential to understand that in a bulk of porous particulate material, two different porosity regimes can be identified: The porosity within the individual particles is referred to as intra-particulate porosity, while the voids that form in between the particles are called inter-particulate pores (Fig. 2). For most drug delivery applications, it is advantageous to load the active substance into the intra-particulate spaces[38], [39].
All of the processes involving accumulating payload in the porous materials entail a loading step. The outcome of the loading process and the final release of the payload depends on the loading method, loading parameters, and the characteristics of the carrier and the loaded substance. These characteristics include particle size, pore diameter, pore-volume, pore shape, and surface area. For example, during the drug loading process, the physical properties of the solvent (e.g., surface tension, viscosity) can influence solvent intrusion into pores. Similarly, the solvent volume used for dissolving the payload determines the initial distribution of the payload in the carrier (i.e., the stronger capillary action facilitates liquid imbibition into smaller pores [40]). Depending on the method and drying rate, the subsequent drying process can also redistribute the payload [28].
Additionally, the physical interactions of the solvent and the drug with the carrier’s surface influence the drug’s initial and final distribution. The region of the carrier where the drug gets deposited and the geometry of the porous structure can eventually also determine the release profile of the drug. Therefore, an interplay of the carrier characteristics and the process parameters can influence the outcome of the drug delivery system.
Despite all the merits of using porous materials as carriers for drug delivery, to our knowledge, an overview of the influence of various parameters on the loading and release of drugs from porous carriers is not available so far. In addition, phenomena such as adsorption and mass transport in porous materials have been vastly explored in other areas where porous materials are used. Much information about porous materials is scattered throughout various research fields, from geology and agriculture to printing technology. A literature review from different disciplines can make this knowledge available for drug delivery. To efficiently employ the full potential of the porous materials for drug delivery, we need to identify the commonly used processes in material loading in porous systems and understand the principles behind such processes.
This article aims to provide a general overview of drug loading and release into and from porous materials, including relevant literature, techniques, related nomenclature, common phenomena, and the physicochemical background of the involved processes. The intention is to present relevant knowledge from disciplines other than drug delivery and to translate this information to be used within the field of pharmaceutical development. Therefore, for many of the terms used interchangeably in the literature, we have used a uniform terminology that is more familiar and applicable to the field of drug delivery (e.g., the term “payload” is used as a substitution for the materials loaded into porous carriers, whether a drug or another compound). Table 1 lists some recent literature that have used porous materials for drug delivery, including some general information for the reader. However, this review does not cover the different types of porous carriers, their production methods, and their applications, as many great reviews exist on these topics [11], [55], [56], [57], [58].
Read more
Maryam Farzan, Roger Roth, Joachim Schoelkopf, Jörg Huwyler, Maxim Puchkov, The processes behind drug loading and release in porous drug delivery systems, European Journal of Pharmaceutics and Biopharmaceutics, Volume 189, 2023, Pages 133-151, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.05.019.
Read more on “Mesoporous Silica: for solubility enhancement of challenging compounds” here:


