Rational Design of Topical Semi-Solid Dosage Forms – How Far Are We?
Specific aspects of semi-solid dosage forms for topical application include the nature of the barrier to be overcome, aspects of susceptibility to physical and chemical instability, and a greater influence of sensory perception. Advances in understanding the driving forces of skin penetration as well as the design principles and inner structure of formulations, provide a good basis for the more rational design of such dosage forms, which still often follow more traditional design approaches. This review analyses the opportunities and constraints of rational formulation design approaches in the industrial development of new topical drugs. As the selection of drug candidates with favorable physicochemical properties increases the speed and probability of success, models for drug selection based on theoretical and experimental approaches are discussed.
This paper reviews how progress in the scientific understanding of mechanisms and vehicle-influence of skin penetration can be used for rational formulation design. The characterization of semi-solid formulations is discussed with a special focus on modern rheological approaches and analytical methods for investigating and optimizing the chemical stability of active ingredients in consideration of applicable guidelines. In conclusion, the combination of a good understanding of scientific principles combined with early consideration of regulatory requirements for product quality are enablers for the successful development of innovative and robust semi-solid formulations for topical application.
Table 1. Overview of qualitative formulation composition of semi-solid topical medicines based on NCE products approved by the FDA from 2016–2022 [22]. The “x” indicates that the respective excipient is contained in the formulation.
Download the full article as PDF here Rational Design of Topical Semi-Solid Dosage Forms-How Far Are We?
or read it here
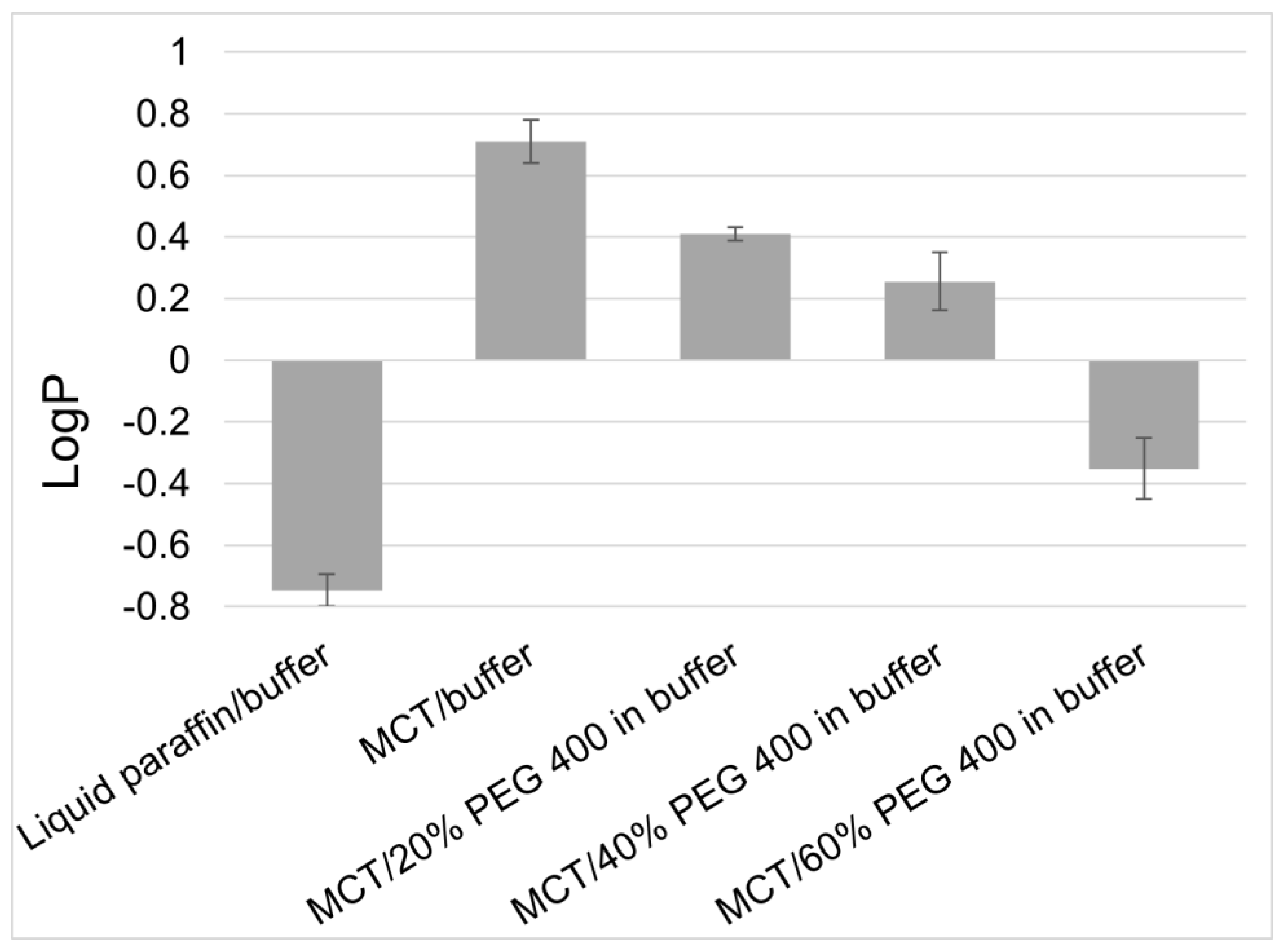
Following excipients are mentioned in the study besides other: Polyethylene Glycol, Polysorbate 20, Polysorbate 80
Herbig, M.E.; Evers, D.-H.; Gorissen, S.; Köllmer, M. Rational Design of Topical Semi-Solid Dosage Forms-How Far Are We? Pharmaceutics 2023, 15, 1822. https://doi.org/10.3390/pharmaceutics15071822
Read more on “Emulsifier” here:


![Table 1. Overview of qualitative formulation composition of semi-solid topical medicines based on NCE products approved by the FDA from 2016–2022 [22]. The “x” indicates that the respective excipient is contained in the formulation.](https://www.pharmaexcipients.com/wp-content/uploads/2023/07/trade1-1024x517.jpg)
![Table 1. Overview of qualitative formulation composition of semi-solid topical medicines based on NCE products approved by the FDA from 2016–2022 [22]. The “x” indicates that the respective excipient is contained in the formulation.](https://www.pharmaexcipients.com/wp-content/uploads/2023/07/trade2-1024x537.jpg)