The step-wise dissolution method: An efficient DSC-based protocol for verification of predicted API–polymer compatibility
Abstract
The development of an amorphous solid dispersion (ASD) is a promising strategy for improving the low bioavailability of many poorly water-soluble active pharmaceutical ingredients (APIs). The construction of a temperature–composition (T–C) phase diagram for an API–polymer combination is imperative as it can provide critical information that is essential for formulating stable ASDs. However, the currently followed differential scanning calorimetry (DSC)-based strategies for API solubility determination in a polymer at elevated temperatures are inefficient and, on occasions, unreliable, which may lead to an inaccurate prediction at lower temperatures of interest (i.e., T = 25 °C). Recently, we proposed a novel DSC-based protocol called the “step-wise dissolution” (S-WD) method, which is both cost- and time-effective. The objective of this study was to test the applicability of the S-WD method regarding expeditious verification of the purely-predicted API–polymer compatibility via the perturbed chain-statistical associating fluid theory (PC-SAFT) equation of state (EOS). Fifteen API–polymer T–C phase diagrams were reliably constructed, with three distinct API–polymer case types being identified regarding the approach used for the S-WD method. Overall, the PC-SAFT EOS provided satisfactory qualitative descriptions of the API–polymer compatibility, but not necessarily accurate quantitative predictions of the API solubility in the polymer at T = 25 °C. The S-WD method was subsequently modified and an optimal protocol was proposed, which can significantly reduce the required experimental effort.
Introduction
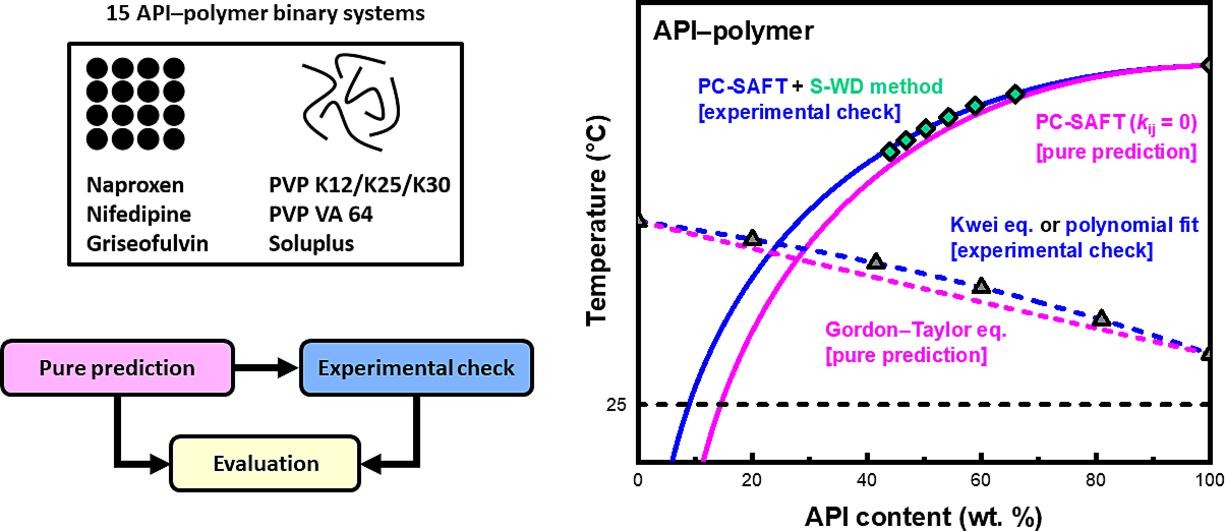
The majority of new chemical entities and a considerable percentage of currently marketed active pharmaceutical ingredients (APIs) are poorly water-soluble, which constitutes a major challenge in the pharmaceutical industry for successful drug product development (Di et al., 2012, Di et al., 2009). To enhance the low oral bioavailability of these APIs, various formulation strategies have been proposed (Gupta et al., 2013). A promising technique is the formulation of a so-called “amorphous solid dispersion” (ASD), whereby amorphous API is molecularly dispersed in a carrier matrix (e.g., a polymer). Several ASD manufacturing techniques have been developed, which can be broadly categorized into solvent evaporation- and melting-based methods (Bhujbal et al., 2021). A prerequisite for successful ASD formulation is favorable compatibility between the API and the polymer so that a homogeneous sample is produced that is either thermodynamically stable or kinetically stable for a pharmaceutically relevant period. Hence, knowledge of the API–polymer temperature–composition (T–C) phase diagram (i.e., the combination of the solid–liquid equilibrium (SLE) (and liquid–liquid equilibrium (LLE)) curve(s) with the glass-transition temperature (Tg) line) is imperative concerning efficient ASD formulation design. However, direct measurement of the API solubility in a polymer at T = 25 °C is challenging, since most polymers are either in the solid state or highly viscous under ambient conditions. Hence, several differential scanning calorimetry (DSC)-based protocols have been established to obtain values at elevated temperatures (i.e., above the API–polymer Tg line), such as the melting point depression (MPD) method (Marsac et al., 2006, Marsac et al., 2009), the recrystallization method (Mahieu et al., 2013), and the zero-enthalpy extrapolation (Z-EE) method (Amharar et al., 2014). The obtained dataset may be subsequently extrapolated to lower temperatures of interest (e.g., T = 25 °C) via an SLE curve modeling approach. An alternative DSC-based protocol called the step-wise dissolution (S-WD) method was recently proposed by our research group as a cost- and time-effective strategy for API solubility determination in a polymer, which can circumvent the aforementioned limitations of the MPD method. In the initial study (Mathers et al., 2021), the MPD, recrystallization, Z-EE, and S-WD methods were compared regarding their ability to determine the solubility of indomethacin (IND) in PVP K12. The MPD, recrystallization, and Z-EE methods were thought to have overestimated the IND solubility due to either unreliable API temperature of melting (Tm) or enthalpy of fusion (ΔfusH) evaluation, or an insufficient time of annealing (ta). Meanwhile, the results obtained via the S-WD method were considered reliable due to the undertaking of a so-called “annealing time investigation” (ATI) experiment, which confirmed that the equilibrium solubility conditions were reached during the applied ta. The S-WD method was successfully modified and applied in a subsequent investigation concerning API solubility determination in a semicrystalline polymer (Mathers et al., 2022).
A major step toward reliable modeling strategies was the arrival of the statistical association fluid theory (SAFT)-type equations of state (EOSs) (Chapman et al., 1989), which were developed in the late 1980s. Built on a solid foundation of statistical thermodynamics, the perturbed-chain statistical association fluid theory (PC-SAFT) EOS (Gross and Sadowski, 2001, Gross and Sadowski, 2002) views molecules as chains of spherical segments that can induce various interactions (e.g., association (i.e., HB), dispersion, multipolar, and repulsion) (Gross and Sadowski, 2002, Gross and Vrabec, 2006). Within the framework of the PC-SAFT EOS, the molecules are completely described by a few sets of physically meaningful pure substance parameters that are typically determined via fitting to experimental values. Over the last decade, numerous studies have been reported in the literature (Dohrn et al., 2021, Lehmkemper et al., 2017a, Lehmkemper et al., 2017b, Luebbert et al., 2017, Prudic et al., 2014a, Prudic et al., 2014b, Prudic et al., 2015) that involves the use of the PC-SAFT EOS concerning ASDs. In the majority of these papers, the author(s) concluded that the PC-SAFT EOS is an effective computational tool for SLE (and LLE) curve prediction with minimal experimental effort. Nevertheless, these seemingly overwhelmingly positive reports may lead other researchers to believe that the general problem concerning unreliable API–polymer compatibility prediction has already been addressed, which is not the case. In these studies, the reported findings via the PC-SAFT EOS typically exclude those related to the pure prediction(s) (i.e., a non-zero binary interaction parameter (kij) is used that was fitted to experimental SLE data). This is most likely due to the pure prediction(s) not being in quantitative agreement with their experimental counterpart(s). Hence, such conflicting results are not included in the publications, which is unrepresentative of the overall ability of the PC-SAFT EOS. Therefore, the underlining potential of the PC-SAFT EOS for reliable kij-free prediction of ASD phase behavior remains an open research topic. Recently, our research group has published studies in which kij-free and kij-tuned predictions of API solubility in a polymer are calculated and compared (Iemtsev et al., 2022a, Pavliš et al., 2023). It should be emphasized that kij-free-based calculations (i.e., pure predictions) are important for expeditious screening of polymeric excipients for a particular API to identify a suitable candidate(s). The favorable purely-predicted SLE curve(s) should then be verified or adjusted using a reliable API–polymer experimental solubility dataset.
In the present work, the ability of the S-WD method to rapidly assess the API–polymer SLE curve modeled via the PC-SAFT EOS for efficient screening of API–polymer compatibility was tested. Five polymeric candidates were considered for three APIs, for a total of fifteen investigated API–polymer binary systems. The PC-SAFT EOS was used to firstly purely predict each API–polymer SLE (and LLE) curve(s), and then secondly each API–polymer SLE (and LLE) curve(s) based on the obtained DSC-based solubility dataset. A comparison between the theoretical- (i.e., kij = 0) and experimental-based (i.e., the optimized kij value) prediction at T = 25 °C was subsequently made. The overall goal of this study was to systematically improve the S-WD method protocol through its application to predominantly simple API–polymer combinations, with the subsequent aim of adapting the main findings for use with more complex systems in future undertakings.
Read more here
Materials
The APIs naproxen (NAP) (CAS registry number (RN) 22204-53-1, GFA class I), nifedipine (NIF) (CAS RN 21829-25-4, GFA class II), and griseofulvin (GRI) (CAS RN 126-07-08, GFA class III) were purchased from Sigma-Aldrich. The polymers Kollidon® 12 PF (PVP K12), Kollidon® 25 (PVP K25), Kollidon® 30 (PVP K30), Kollidon® VA 64 (PVP VA 64), and Soluplus® (SOL) were kindly donated by BASF.
Alex Mathers, Matouš Pechar, Fatima Hassouna, Michal Fulem, The step-wise dissolution method: An efficient DSC-based protocol for verification of predicted API–polymer compatibility, International Journal of Pharmaceutics, Volume 648, 2023, 123604, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123604.
Read also more on Orally Disintegrating Tablets (ODTs) here:


