A study of the impact of excipient shielding on initial drug release using UV imaging
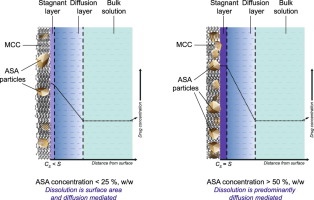
Knowledge on the dissolution behaviour of a drug is critical for efficient and effective product development. As the drug has almost always to be formulated with excipients in the design of a dosage form, it is important to examine the implications of the choice of excipients on the dissolution of the drug, among others, especially in the case of an immediate release dosage form. The objective of this study was to explore the potential of using an ultraviolet (UV) imaging technique to examine the effect of drug-excipient ratio on the initial dissolution of the drug, when formulated with a hydrophilic, water insoluble excipient. A series of drug-excipient binary blends with different ratios were prepared and compacted into 2 mm compacts, and their dissolution profiles captured with a UV imager. Chemical imaging via Raman spectroscopy was also performed on the compacts to quantify the fraction of drug presented on the compact surface. At low drug concentrations, a suppression in drug dissolution was observed, but beyond a critical drug-excipient ratio, the concentration of the excipient no longer played a role in affecting drug dissolution rates. Drug particle size was found to affect the critical drug-excipient ratio required to negate the shielding effect exerted by the excipient, such that a higher proportion of drug was required. It is postulated that the excipient served as a physical barrier, as well as competitor for water required for wetting during initial dissolution, thereby causing a delay in the wetting and dissolution of the drug.

