Effect of melt extrudability and melt binding efficiency of Soluplus® on release pattern of hydrophilic and high dose drugs
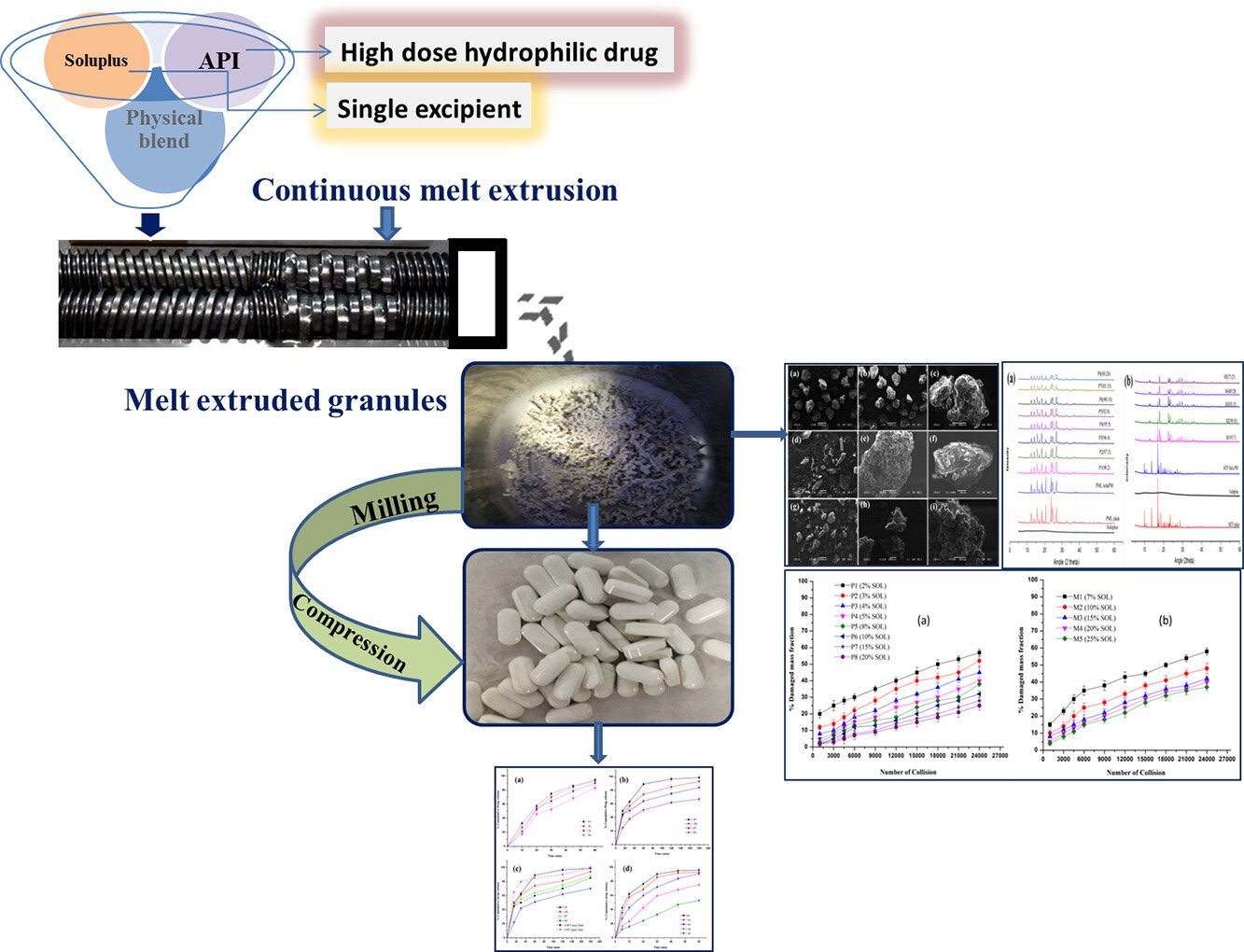
The present study explores the effect of melt binding of Soluplus® on in vitro release profiles of two hydrophilic drugs, metformin hydrochloride, and paracetamol. The melt viscosities of bulk polymer and physical mixtures with different concentrations of selected APIs were analyzed by using a rheometer. The rheological evaluation revealed both the suitable temperature range for melt extrusion process and drug-polymer extrudability. The effect of formulation and processing parameters (e.g. polymer/drug ratio, temperature, screw speed) on extrudability were evaluated in terms of torque and residence time analysis.
The extrudates obtained via hot melt extrusion (HME) processing exhibited good flow and compressibility. Differential scanning calorimetry (DSC) and X-ray diffraction studies examined the change in glass transition temperature (Tg) and crystalline pattern of extruded formulations where all extruded formulations seemed to have retained their crystallinity. The thermogravimetric analysis determined the thermal stability (weight loss) as a function of operating temperature whereas scanning electron microscopy (SEM) showed agglomerated microstructure and rough surface with a porous network and void spaces.
The tablets obtained after compression of milled extrudates showed excellent hardness with robust tablet characteristics. The in vitro release studies of individual batches performed in various USP recommended dissolution media (for paracetamol) showed the pH-independent release of the hydrophilic drugs from the polymer matrices. Continue reading on Soluplus and extrusion

