Creation of Filaments for 3D Printing via Hot Melt Extrusion: Evaluating Different Downstream Technologies
Purpose: For several years now 3D printing technology has been gaining increasing attention within the pharmaceutical industry. The number of publications is rapidly increasing. One promising 3D printing technology is the fused deposition modeling where a polymer strand is heated and extruded through a small nozzle followed by a solidification on a build plate. The production of the filaments itself is already described, but so far low focus is laid on assessing the filament properties like varying filament diameter or break tendencies. Our main goal was therefore to evaluate the production of highly loaded ( >15% API load), highly homogeneous filaments which shall provide excellent properties for 3D printing applications.
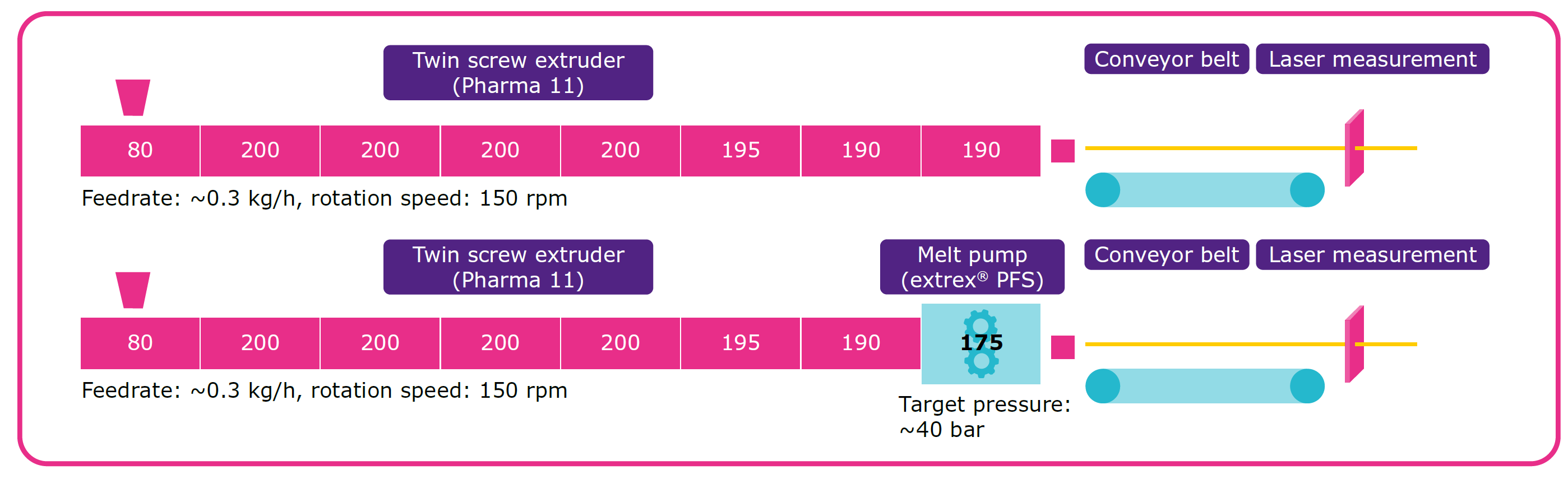
Objectives: The aim was to produce a Parteck® MXP based filament with minor variation in filament diameter and superior 3D printing capabilities as well as a significant amount of API content. Different downstream technologies were assessed in order to optimize the filament geometry and homogeneity. A 3-axis laser measurement device was used as an online in process control in order to assess the homogeneity of the filament over the entire production.

Methods: Polyvinyl alcohol based Parteck® MXP excipient from Merck KGaA (Darmstadt, Germany) was used as a polymer for the hot melt extrusion. The model drug substance ketoconazole was purchased from LGM Pharma (Erlanger, KY 41018, USA). Filaments for 3D printing with a target diameter of 1.75 mm were produced with an extrex® PFS – 20GP Melt pump from Maag Automatik GmbH (Groß-Ostheim, Germany) attached to a Pharma 11 twin-screw extruder from Thermo Scientific (Karlsruhe, Germany). The diameter of the filaments was measured with a 3-axis laser micrometer from Zumbach (Orpund, Switzerland) ODAC Trio33. An overview of the filament manufacturing process is presented in Figure 1. 3D-Printing was performed using an Ultimaker 3 (firmware version: 4.0.1.20171023, Ultimaker, Geldermalsen, Netherlands), which was modified to enable printing of filament with a diameter of 1.75 mm. Nozzle diameter was 0.4 mm. Tablets were designed in Fusion 360 (Autodesk, Farnborough, United Kingdom). Therefore, a cylinder shape was designed (diameter: 10 mm, height: 2.4 mm). Simplify 3D (version 4.0.1. Simplify3D, Cincinnati, USA) was used for slicing. Tablets were printed at 10 mm/s with an infill density of 100%. All printing parameters were kept equal, except for printing temperatures. Parteck® MXP was printed at 230 °C and Ketoconazole containing filaments at 210 °C.
Results: The Integration of a melt pump reduces the pulsation of the melt and enhances the precision of the targeted filament geometry. It can be shown that the use of a melt pump can increase the consistency and stability of the process. All printed samples show a homogenous weight distribution of the tablets. In our first assessment the geometry of the tablets was not affected by the different filament manufacturing techniques. Independent from drug load or the respective production method very reliable results could be achieved.
Conclusion: Identifying the right down-stream equipment can be an important factor for creating a homogenous filament. The use of a melt pump can improve the consistency and ensures the safety of the process.
During production the pulsation of the melt flow could be drastically reduced. Also a positive impact on the filament geometry can be assured.
For the evaluated samples both technologies pro-vided reliable material properties for 3D printing. Variations in diameter were observed at an extend where the geometry of the final dosage form was not negatively affected.
It can be expected that at a larger production scale the benefits of a melt pump are even more pronounced providing the basis for a further automation of the process.
 Download the full poster as a PDF here
Download the full poster as a PDF here
Authors: T. Kipping, C. Makert, M. Richter, A. Geissler-Fichtner, N. Gottschalk, N. Di Gallo, A. G. Elia, A. N. Knuettel, F. Bauer.


