Smart pills and drug delivery devices enabling next generation oral dosage forms
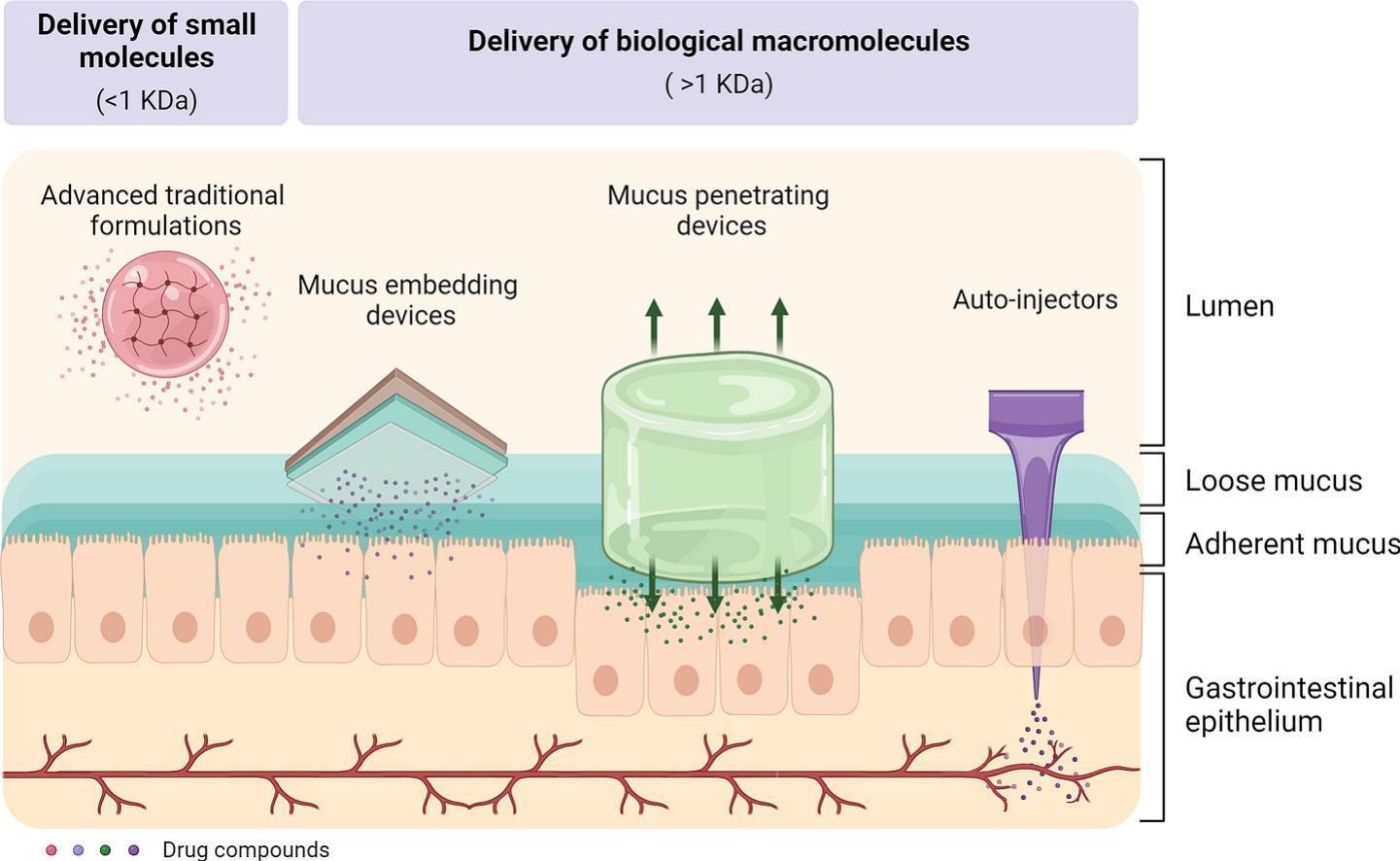
Oral dosage forms are the preferred solution for systemic treatment and prevention of disease conditions. However, traditional dosage forms face challenges regarding treatment adherence and delivery of biologics. Oral therapies that require frequent administrations face difficulties with patient compliance. In addition, only a few peptide- and protein-based drugs have been commercialized for oral administration so far, presenting a bioavailability that is generally low. Therefore, research and development on novel formulation strategies for oral drug delivery has bloomed massively in the last decade to overcome these challenges. On the one hand, approaches based on lumen-release of drugs such as 3D-printed capsules and prolonged gastric residence dosage forms have been explored to offer personalized medicine to the patient and reduce frequent dosing of small drug compounds that are currently in the market as powdered tablet or capsules. On the other hand, strategies based on mucus interfacing such as gastrointestinal patches, or even epithelium injections have been investigated in order to enhance the permeability of biologic macromolecules, which are mostly commercialized in the form of subcutaneous injections. Despite the fact that these methods are at an early development stage, promising results have been revealed in terms of personalized medicine and improved bioavailability. In this review, we offer a critical overview of novel ingestible millimeter-sized devices and technologies for oral drug delivery that are currently used in the clinic as well as those that could emerge on the market in a not too distant future.
1. Introduction
Providing the right dose at the right place at the right time is the ideal aim for treatment and prevention of disease conditions – and ingestible dosage forms remain the preferred solution for that purpose [1]. However, traditional oral dosage forms face challenges which warrants the continuous development of novel drug delivery technologies [2]. First, commercialized oral drug dosage forms are primarily produced by tableting, and here equipment restrictions limit the fabrication of single unit dosage forms capable of delivering e.g. multiple drugs with precise doses and release profiles according to the individual needs of the patient [3,4]. Secondly, there is a lack of patient adherence to therapies that require frequent administrations of one or multiple medicines such as HIV antiretroviral therapies [5]. There are available technologies that aim to overcome this challenge such as Osmotic-controlled Release Oral delivery Systems (OROS), which have been on the market for dozens of years and rely on the principle of osmosis as the driving force for sustained release of pharmacotherapy, reducing the dose frequency to once-daily [6,7]. However, there is still a lack of adherence to daily medications, and therapies that need a weekly or monthly sustained release are limited by the short residence time of traditional dosage forms [[8], [9], [10], [11]]. Finally, there are challenges associated with a low bioavailability of macromolecules such as peptides when using traditional oral dosage forms, due to low mucosal/epithelial permeability and lack of stability in the gastrointestinal (GI) environment [12,13]. The recent commercialization of semaglutide highlights the incorporation of permeation enhancers (PEs) as a way to increase absorption of orally delivered macromolecules [14,15]. However, the oral bioavailability achieved for peptides, even with the PEs that have been commonly tested in clinical studies, is generally low [16,17]. The current state-of-the-art technology for a successful oral peptide delivery provides around 1% bioavailability when delivered as a standard oral tablet utilizing salcaprozate sodium (SNAC) as PE (Rybelsus® oral semaglutide) [14].
These challenges suggest a need to develop novel oral dosage forms that adapt to the specific requirements of the patient and the pharmaceutical industry and to identify methods of improving adherence in the patient populations. Moreover, there is a need to develop oral formulations that protect and enhance the permeability of macromolecules.
The aim of this review is to offer a focused and critical analysis of novel millimeter-scale oral drug delivery technologies for systemic uptake, including smart pills and ingestible engineered devices that are currently used in the clinic or are in preclinical trials, and as well as those that could emerge on the market in a not too distant future. It is considered outside the scope of this review to summarize nano- and micro-particle systems for oral drug delivery. Therefore, readers interested in this topic are suggested to look elsewhere [[18], [19], [20]]. More explicitly, we have focused on engineered formulations for controlled release of pharmaceutics in the lumen such as 3D-printed capsules and prolonged gastric residence devices, which aim to delivery small drug compounds, as well as devices that enhance the bioavailability of biologic macromolecules by mucus embedment/penetration or even epithelium injections using needle-based devices and auto-injectors (Fig. 1).
Download the full article as PDF here: Smart pills and drug delivery devices enabling next generation oral dosage forms
or read it here
Carmen Milián-Guimerá, Reece McCabe, Lasse Højlund Eklund Thamdrup, Mahdi Ghavami, Anja Boisen, Smart pills and drug delivery devices enabling next generation oral dosage forms, Journal of Controlled Release, Volume 364, 2023, Pages 227-245, ISSN 0168-3659,
https://doi.org/10.1016/j.jconrel.2023.10.041.

