Novel Excipients: Gaining Traction in the Industry

By Patricia Van Arnum – DCAT Editorial Director – August 5, 2020
The FDA is considering a pilot program for the toxicological and quality evaluation of novel excipients. Excipients now are not separately reviewed by the FDA, and some say a separate review mechanism would support development of new excipients and overall product innovation.
Novel excipients and the FDA
Currently, novel excipients are not precluded a regulatory filing (investigational new drug application (IND), new drug application (NDA), or biologics license application (BLA)), but in practice, pharmaceutical companies are reluctant or do not include new excipients in their applications over concerns that inclusion of a new pharmaceutical ingredient other than the active ingredient in a drug formulation may raise additional regulatory concerns and possibly jeopardize a drug filing. Proponents of an FDA novel excipient review program say that the FDA’s recognition of a novel excipient would reassure drug developers that the novel excipient can be used in a drug-development program while minimizing the risk that safety concerns would be raised by the FDA during application review. They have also cited a perceived risk aversion on the part of drug developers, such that novel excipients may be avoided in drug-development programs, even when the excipients have potential health benefits. A novel excipient review program, therefore, would allow companies to know in advance if a new excipient was acceptable from a regulatory view without running the risk of an IND, NDA, or BLA being rejected or delayed due to questions over a novel excipient. Adjusting the regulatory review process as such, some say, would encourage the development of novel excipients, which is important to provide pharmaceutical companies and excipient manufacturing the needed flexibility to develop new excipients as may be needed to resolve drug-formulation changes.
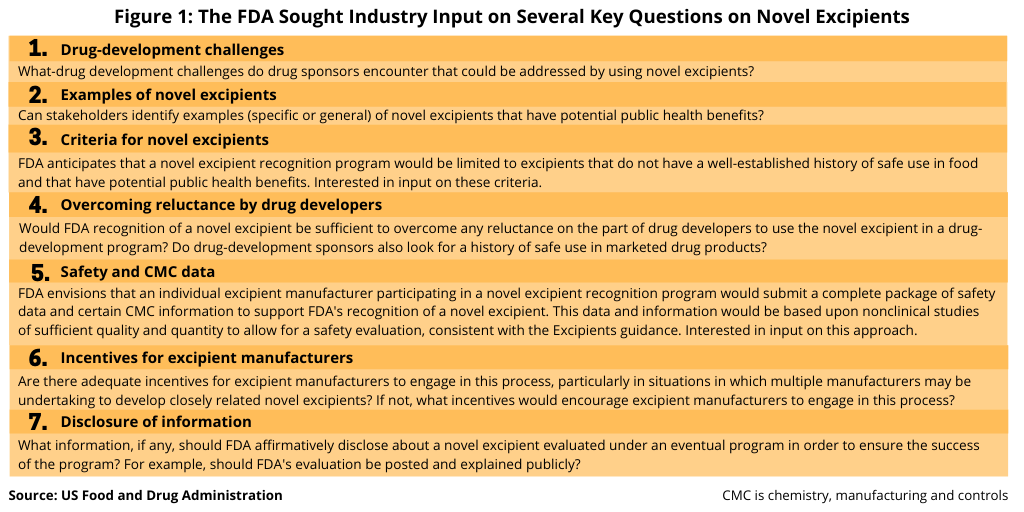
The US Food and Drug Administration (FDA) took a first step in considering such an approach late last year (December 2019) by issuing a request for input from the industry for purposes of obtaining information and comments that will assist the agency in determining whether it should establish a pilot program for the toxicological and quality evaluation of novel excipients intended for use in human drugs. The agency was interested in obtaining information and comments on several aspects of such a program before deciding whether to develop it. The industry had until early February (February 2020).
The FDA was interested in getting input from the industry on several key questions, including would such an approach overcome drug developers’ reluctance in using novel excipients and what criteria should be required for the novel excipient.