FTIR and TG analyses coupled with factor analysis in a compatibility study of acetazolamide with excipients
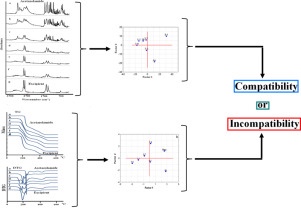
A compatibility study of drug substance with excipients is a crucial step in the drug development process in order to generate potent final drug formulations for efficient and safe therapy for various diseases. Thus, the development of new methods for compatibility studies is a great challenge. For this reason, a new approach based on improvement of FTIR spectra and TG curves interpretation using factor analysis (FA) was developed as a screening technique for assessing the compatibility of acetazolamide with selected excipients. This multivariate method demonstrates that in some cases acetazolamide and mixtures with high acetazolamide content formed one cluster, while a second cluster consisted of excipient and mixtures with high excipient content. Such clustering of the analyzed samples (drug substance, excipient and their mixtures) demonstrates the compatibility between ingredients. This in turn means that the FTIR spectra and TG curves of mixtures are the sum of absorption bands or of the thermal profiles of ingredients. In the case of incompatibility, the FTIR spectra and TG curves of mixtures differ from those of ingredients. The FA score scatter plot shows that clusters consist of mixtures which differ with respect to ingredient content. In conclusion, FA proved the incompatibility of acetazolamide mixtures with β-cyclodextrin, chitosan, lactose, mannitol, meglumine and starch. This was also confirmed by complementary techniques such as DSC and PXRD. Hence, the application of FA can be helpful for better comprehension of data obtained from FTIR spectra and TG curves.

