Amorphous – state Characterization and Dissolution Behaviour of Efavirenz – Tocopheryl Polyethylene Glycol Succinate (TPGS)-1000 Solid Dispersions
ABSTRACT
The aim of the present investigation was to enhance the aqueous solubility and dissolution rate of a poorly soluble drug, efavirenz, by preparation of solid dispersions with tocopheryl polyethylene glycol succinate (TPGS)-1000, a non-ionic surfactant. Phase solubility studies suggested that the solubility of efavirenz increased with the increase in TPGS concentration.1 absorption. Gibbs free energy (ΔG) were negative, indicating the spontaneous nature of efavirenz solubilization. Dispersions in different ratios were prepared using the fusion method and their physicochemical characteristics were investigated using Fourier Transform Infrared Spectroscopy(FTIR), X-ray Powder Diffraction studies(XRPD) and Scanning Electron Microscopy(SEM).A drug: carrier ratio of 1:1.5 w/w prepared by cooling the fused mixtures at 5° C showed the highest drug release of 33.27%.It was demonstrated that decrease in crystallinity of the drug and H-bonding between efavirenz and TPGS-1000 might be responsible for the enhanced dissolution rate. Analysis of dissolution data showed the best fitting with Higuchi model.
INTRODUCTION
Efavirenz [(S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1- benzoxazin-2-one] (molecular weight 315.68) , a HIV – type I specific non-nucleoside reverse transcriptase inhibitor (NNRTI) (Balani et al.,1999) ,is used in the treatment of HIV-1 infections at a usual adult dose of 600 mg once a daily (Csajka et al.,2003). It is a crystalline lipophilic solid with a low aqueous solubility of 0.01 mg/ml and a low intrinsic dissolution rate of 0.037mg/ cm2 /min3. An intrinsic dissolution rate less than 0.1 mg/ cm2 /min (Parvin et al.,2009) is a rate limiting factor for oral drug absorption(Kaplan,1972) It is classified under BCS class II according to the biopharmaceutical classification system by FDA (Kasim et al.,2004).Futhermore, It has very poor and variable absorption (40-45%) due to extremely low aqueous solubility. Efavirenz is currently administered in higher dose in oral dosage for, hence there is need to alleviate its dissolution rate limited absorption problem by enhancing its dissolution rate and thus achieve dosage reduction to achieve desired therapeutic effect.
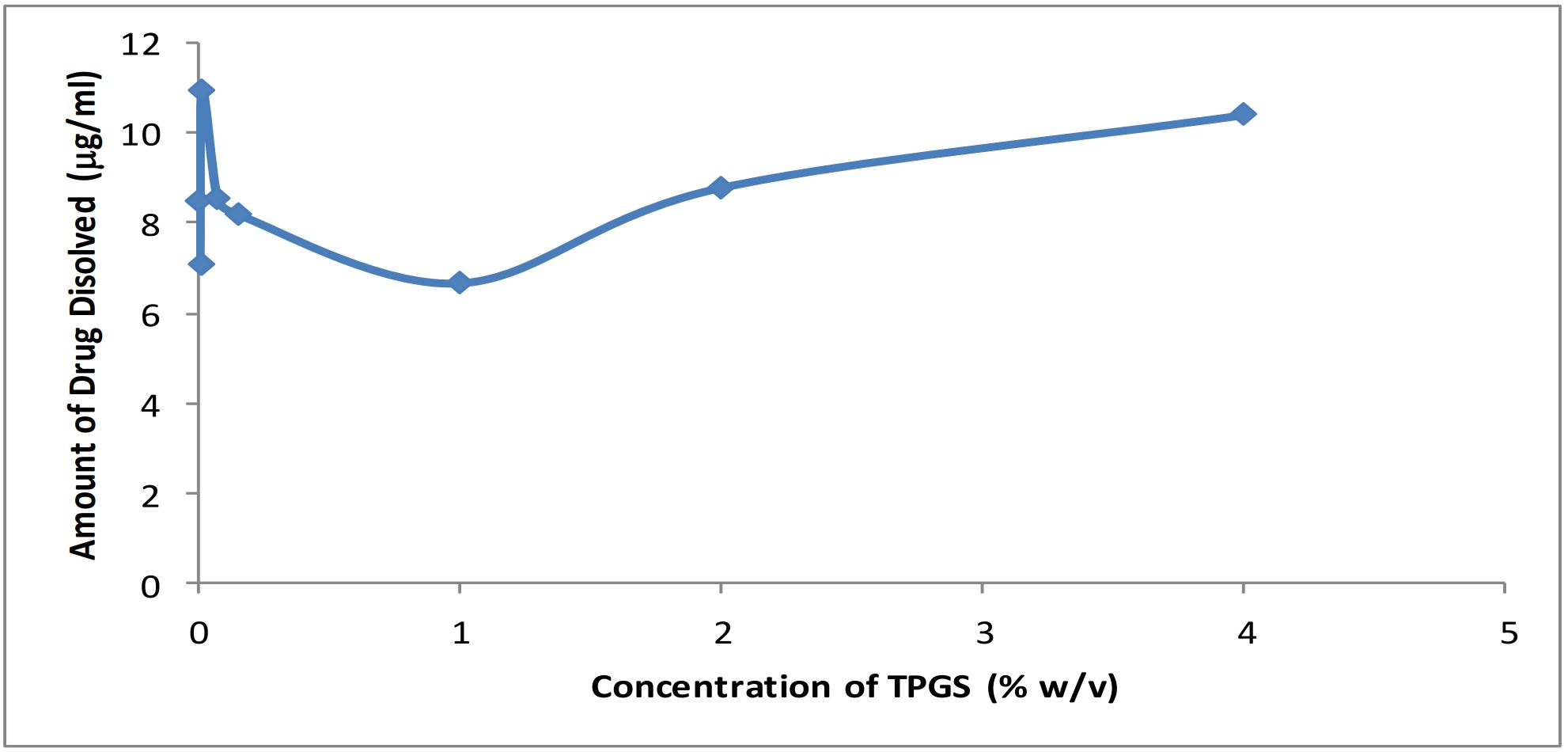
TPGS, a D-alpha vitamin E ester derived from natural vitamin E, consists of mixture of monoester, certain diester and residual free PEG -1000.A non-ionic surfactant with HLB value of 13.2, it is used as a emulsifier, stabilizer, absorption enhancer and a controlled release agent in pharmaceutical formulations. It improves the solubility of lipophilic compounds through micelle solubilization above its critical micelle concentration (CMC) of 0.1 mM (Eastman chemical publication,1998).TPGS-1000 has been successfully used as solubility enhancer for furosemide (Shin and Kim,2003); to improve the bioavailability of cyclosporin and amprenavir substantially by enhancing their solubility and permeability (Yu et al.,1999) (Ismailos et al.,1994) ; to enhance oral absorption of cyclosporin A by improved solubilization through micellization in liver transplant patients (Sokol et al.,1991) and to enhance the aqueous solubility and absorption of paclitaxel (Varma and Panchagnula,2005) In the previous studies, the dissolution rate of efavirenz was enhanced using carriers like PEG 6000, poloxamer 407, carrageenan and by cyclodextrin complexation.
In the present investigation, solid dispersions of efavirenz with TPGS 1000 as a carrier were prepared using the fusion method. The objective of the current work was to evaluate the feasibility of formulating a solid dispersion of efavirenz with enhanced solubility/dissolution rate. The joint influence of ‘drug to carrier ratio’ and ‘temperature to which drug-carrier fused mixture was cooled’ was studied on the drug release. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRPD), scanning electron microscopy (SEM) and dissolution tests were employed to characterize the prepared solid dispersions.
Download the full article as PDF here: Amorphous –state Characterization and Dissolution Behaviour of Efavirenz
or read it here
Materials
Efavirenz was obtained as a gift sample from Ranbaxy Laboratories Pvt. Ltd., Paonta Sahib.TPGS-1000 was obtained from Sigma Aldrich, Mumbai, India. All other reagents and solvents (Rions, India) used were of analytical grade. Double distilled water and triple distilled water were used throughout the studies.
JASMINE KAUR RANDHAWA, RABIA DHILLON, & NEENA BEDI. (2024). Amorphous –state Characterization and Dissolution Behaviour of Efavirenz- Tocopheryl Polyethylene Glycol Succinate (TPGS)-1000 Solid Dispersions. International Journal of Pharmacy Research & Technology (IJPRT), 14(2), 1–12. Retrieved from https://www.ijprt.org/index.php/pub/article/view/243
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


