Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes – A proposed roadmap
The APV presents the scientific paper recently published by the APV Task Force Patient Centric Medicine (PaCeMe):
Abstract
The increasing awareness of acceptability and usability of pharmaceutical drug products by the patient as a key quality requirement continues to drive need for integrating patient centric drug product design into the pharmaceutical development process. The complex matrix of multiple drug product related decisions during the early drug development process often limits patient-centric drug product (PCDP) design options in the final commercial drug product development phase. To integrate the specific needs and perspectives of patients into drug development and product design process, a rational approach integrated into the complex development matrix is required from the start and weighs product development decision options accordingly. The aim of this work was to develop a roadmap for PCDP design in a multidisciplinary approach that leads to better usability, adherence and acceptance of the drug by patients via early integration into the development matrix. The proposed rational approach is based upon regulatory requirements and lessons learned from pediatric and geriatric drug development.
Download the full paper as PDF: Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes – A proposed roadmap
1. Introduction
Due to progress in pharmacotherapy and the resulting variety of therapeutic options, as well as the social development towards increasing personal responsibility and individuality, the role of the patient and care giver ecosystem has steadily evolved. From a rather passive role, patients are more and more taking an active part in the decision-making process as they are the most critical factor in the implementation of therapy and physicians increasingly implement shared decision making on the therapeutic approach [1]. Without the active and continued contribution of the patient, the product might not be taken properly and hence therapeutic outcomes are likely to be sub optimal.
The provision of safe and effective pharmaceutical drug products to be used and administered by the patients, care givers and healthcare professionals have to fulfill a number of important criteria to display the best benefit-to-risk profile. Besides the efficacy and safety of the drug compound, which is essential for the clinical outcome, the finished pharmaceutical drug product needs to be of high quality and stability, affordable cost, accessible as well as acceptable and usable by the end user. Over the past decades, evidence is emerging on the importance of the acceptability and usability of pharmaceutical drug products and the contribution this might have to non-adherence, medication errors, other medication related problems and undesired therapeutic outcomes. While the problems in dealing with existing pharmaceutical products are well described [2], [3], [4], our knowledge regarding optimal drug product design and the implementation of patient-centric drug product design [5] within existing regulatory/clinical guidelines remains very limited [6], [7], [8]. However, there is increasing scientific and regulatory research on the patient perspective throughout the development process of new therapies [9], [10] including acceptability and usability of the drug product. These issues have already been recognized for the pediatric patient population, but are equally acute for other patient populations such as geriatric or multimorbid patients [11].
Considering the complexity of the matrix of a new drug product development, the intrinsic physico-chemical and pharmacological properties of a drug compound, the variety of different patient characteristics and needs, the existing regulatory and industrial requirements as well as the timely access for patients to effective new therapies, the integration of patient-centric pharmaceutical product design remains a major challenge [8], [12]. In addition, the multiplicity of different stakeholders, tasks and deliverables, the need for scientific evidence of patient-centric drug product development and design as well as the lack of regulatory guidelines, are challenges for cross-disciplinary discussions and joint research. To overcome these barriers, a pragmatic, rational, and stepwise process commonly agreed between different stakeholders and development organizations is required. One such approach is suggested in this paper. Since each drug development program is specific to the disease, the drug compound and the intended patient population, the process considered must be general in nature to serve as a RoadMap. It must be able to justify the scientific approach taken during development of the patient-centric drug product, including the rationale for product design decisions and associated risk mitigation during the early development stages and scale-up. While there are probably no solutions to all problems yet, a better solution can be developed for every product, through a RoadMap that will improve the product design by qualified assumptions to support decision making without compromising equally important quality requirements of the final drug product, its approval and patient access. The RoadMap can also be applied in life cycle management to further improve the product design or better serve additional patient population.
This publication is intended to serve as a proposal for a RoadMap towards patient-centric drug product design to stimulate further input and scientific discussions with other experts and stakeholders across all disciplines.
2. Methods
The Patient Centric Medicine Initiative (PaCeMe) is a multidisciplinary stakeholder group of academic and industrial individuals with specific expertise covering various aspects of drug development and the interface of patients with their medical products.
After reaching consensus that the complexity of the subject as well as the development matrix of a new drug product will require a case-by-case approach, it was acknowledged that it would not be possible to provide a single, generally applicable RoadMap. However, there was consensus that a RoadMap can be developed that provide a logical framework and process to steer identified patient needs towards inclusion into PCDP development and design. For the RoadMap development, the following preliminary considerations were defined:
- • Leverage prior experience and work done in similar field (e.g., product development and clinical supplies in pediatrics, geriatrics)
- • Use of available information related to patient characteristics and needs in disease or discipline specific guidelines, documents or publication (screening of literature and regulatory guidance)
- • Follow the principles of a rational, meaningful and practical approach, taking into account the feasibility of implementation
- • Define knowledge gaps, methodological limitations, and issues with qualification and quantification of results to be addressed in future research
- • Assure all stakeholder inclusion and users involvement in the RoadMap for product development and life cycle
- • For practicality reasons, the framework development was limited to oral dosage forms, but should be adaptable and applicable to other dosage forms as well
Based on the preliminary considerations a screening of the literature and regulatory guidance documents relevant to the RoadMap was performed and analyzed. In addition, subgroups were formed to collect insights on (1) patients and therapeutic conditions; (2) acceptability; (3) formulation and manufacturing; and (4) market access and prescribing with a focus on type 2 diabetes mellitus. This information served RoadMap the objective to demonstrate the practicality of the RoadMap as a case study.
3. Results
3.1. Screening of literature relevant to the RoadMap
A screening of the literature identified approaches developed within the pediatric drug product development space. Severin et al described the importance of forming a Pediatric Expert Group within the company to enhance internal collaboration and external collaboration with e.g., research networks, academic institutions and public private partnerships to enhance the quality and efficiency of pediatric medicines development [12]. Sam et al proposed a framework for the development of drug products for pediatric populations. The framework includes considerations for the selection of age-appropriate dosage forms and how to make use of existing formulation approaches and technology to the benefit of the adult populations as well [13]. The WHO and Unitaid published guidance on the clinical and pharmaceutical development of pediatric antiretroviral drugs consisting of 10 modules. Modules 5, 6 and 7 provide useful information about the state-of-the-art knowledge, gaps and potential solutions to assess and achieve acceptability (Module 5), to involve patients and their community (Module 6) and define an appropriate Target Product Profile [14]. Additionally, precompetitive consortia have been established in Europe like e.g. the European Paediatric Formulation Initiative (EuPFI) that aims to resolve scientific, regulatory and technological issues associated with paediatric formulation development or the European Paediatric Translational Research Infrastructure (EPTRI) which aims to accelerate the pediatric drug development [https://eptri.eu/].
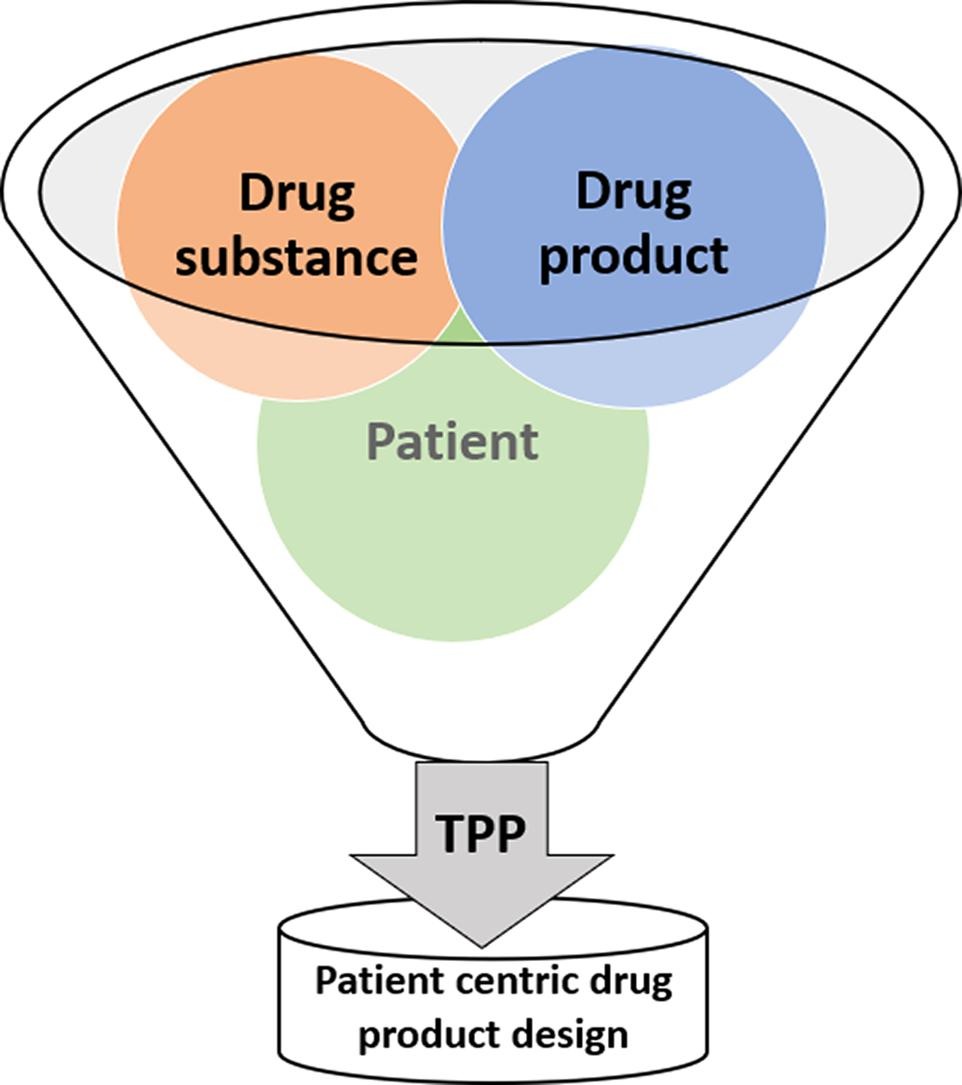
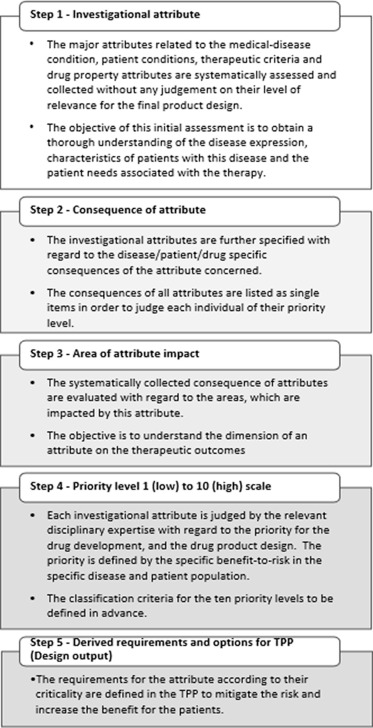
The proposed definition of patient-centric pharmaceutical drug product design describes a 3-step approach. Four different factors were identified as design drivers in the first step (characteristic of disease/condition, characteristic of drug substance/physiology, characteristic of drug therapy, characteristic of drug product, patient characteristics) for which design inputs and design outputs can be described in the second and the third step respectively to guide the drug product development process [5].
Human factor engineering and ergonomic methods for medical device development provide a framework to assess the product-user interface and to assure a smooth and error-free interaction between the product and the user by an iterative design process with direct user involvement [15].
A design process can also be guided by a heuristic problem-solving approach, building on the ability and intuition of the users when interfering with a drug product with the goal of ensuring ease of use without user instructions. A three-step process was proposed, (1) define how the product should be used, (2) identify the most serious challenges of the usage and any resulting complications that must be avoided and (3) identify solutions that support users best while minimizing the cognitive effort required to use the product. The heuristic approach can be used prospectively based on existing knowledge and retrospectively by gaining more knowledge for further product improvements [16].
Download the full paper as PDF: Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes – A proposed roadmap
Sven Stegemann, Liz Sheehan, Alessandra Rossi, Andrew Barrett, Amrit Paudel, Abina Crean, Fabrice Ruiz, Massimo Bresciani, Fang Liu, Zakia Shariff, Margarete Shine, Christel Schmelzer, Anne-Marie Pense-Lheritier,
Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes – A proposed roadmap, European Journal of Pharmaceutics and Biopharmaceutics, Volume 177, 2022, Pages 81-88, ISSN 0939-6411

