Solid self-emulsifying casein carrier for the improvement on the oral bioavailability of simvastatin
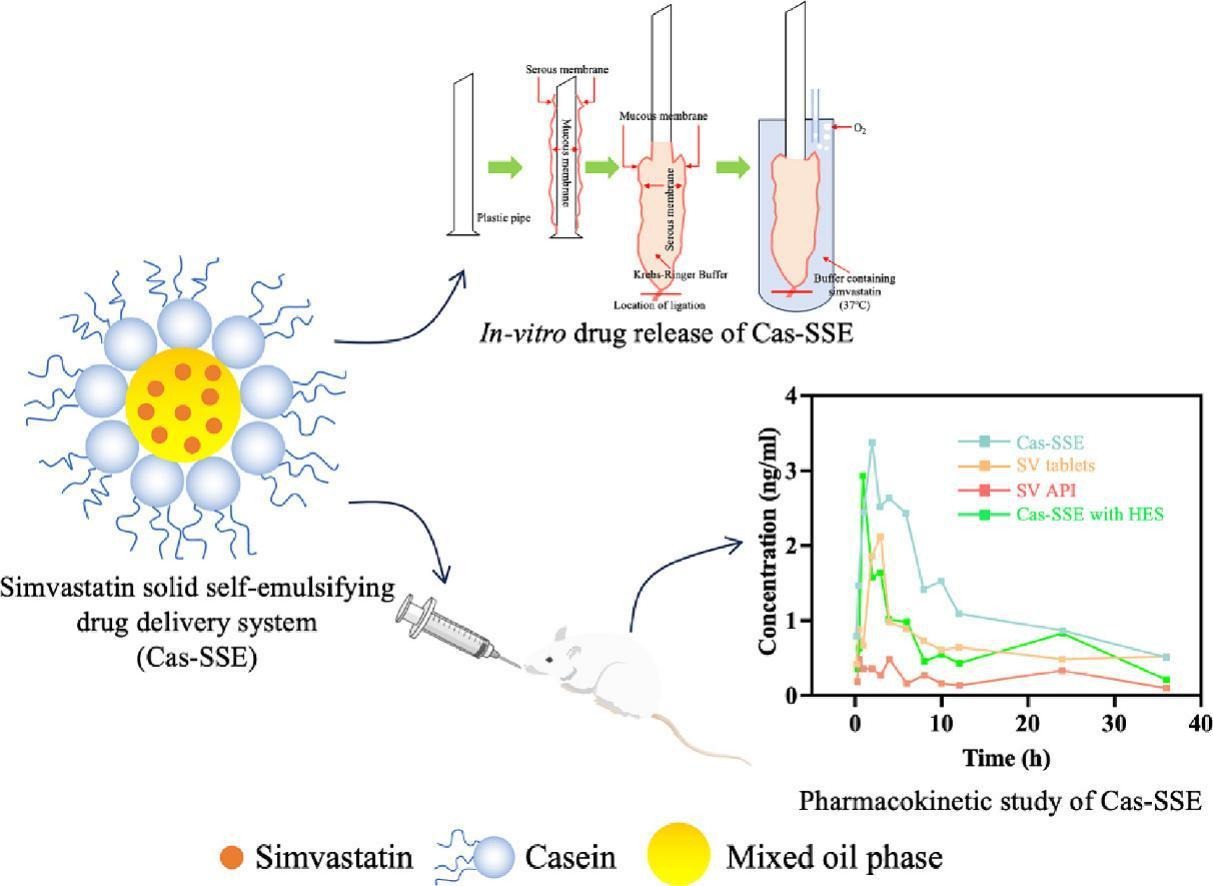
Simvastatin (SV) is a statin drug that can effectively control cholesterol and prevent cardiovascular diseases. However, SV is water-insoluble, and poor oral bioavailability (<5 %). Solid self-emulsifying carrier system is more stable than liquid emulsions, facilitating to improve the solubility and bioavailability of poorly soluble drugs. In the present study, a solid self-emulsifying carrier stabilized by casein (Cas-SSE) was successfully used to load SV to improve its solubility in water, by formulation selection and emulsification process optimization.
Compared with oral tablets, the release of SV from Cas-SSE was significantly enhanced in artificial intestinal fluid. Furthermore, everted gut sac experiments indicated some water-soluble dispersing agents such as hydroxyethyl starch (HES), were not conducive to drug absorption. Pharmacokinetic studies suggested Cas-SSE without dispersing agent has much higher relative bioavailability (184.1 % of SV and 284.5 % of simvastatin acid) than SV tablet. The present work suggests Cas-SSE is a promising drug delivery platform with good biocompatibility for improving oral bioavailability of poorly water-soluble drugs.
Read more here
Materials
SV tables (drug loading 14.24 %) was purchased from Beijing Fuyuan Pharmaceutical Co., Ltd., Beijing, China. SV active pharmaceutical ingredient (API) was purchased from Zhejiang Haizheng Pharmaceutical Co., Ltd., Zhejiang, China. Casein was purchased from Gansu Hualing Casein Co., Ltd., Gansu, China. Hydroxyethyl starch (HES, 200 KDa) was purchased from Wuhan Life Technology Co., Ltd., Hubei, China. Capryol 90, maisine 35–1, and WL 1349 were purchased from Gattefossé, Lyon, France and Soybean oil.
Han Li, Haixia Sun, Yanbing Zhao, Shaobin Wang, Yongsheng Zhao, Solid self-emulsifying casein carrier for the improvement on the oral bioavailability of simvastatin, International Journal of Biological Macromolecules, 2024, 131516, ISSN 0141-8130, https://doi.org/10.1016/j.ijbiomac.2024.131516.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


