Aerogels as Carriers for Oral Administration of Drugs: An Approach towards Colonic Delivery
Polysaccharide aerogels have emerged as a highly promising technology in the field of oral drug delivery. These nanoporous, ultralight materials, derived from natural polysaccharides such as cellulose, starch, or chitin, have significant potential in colonic drug delivery due to their unique properties. The particular degradability of polysaccharide-based materials by the colonic microbiota makes them attractive to produce systems to load, protect, and release drugs in a controlled manner, with the capability to precisely target the colon. This would allow the local treatment of gastrointestinal pathologies such as colon cancer or inflammatory bowel diseases. Despite their great potential, these applications of polysaccharide aerogels have not been widely explored. This review aims to consolidate the available knowledge on the use of polysaccharides for oral drug delivery and their performance, the production methods for polysaccharide-based aerogels, the drug loading possibilities, and the capacity of these nanostructured systems to target colonic regions.
1. Introduction
The oral administration route is the most common approach for the local and systemic therapeutic treatments of a wide range of pathologies [1,2,3]. It is the natural physiological pathway to incorporate nutrients into the body and the easiest way to administer drugs. Active compounds can be absorbed in three different sections of the gastrointestinal tract (GIT): the stomach, small intestine, and large intestine. The stomach is a structure specialized in decompounding the ingested food, but its capability for the absorption of drugs is limited. The small intestine is the section specialized in nutrient absorption due to its tremendous surface, where the drugs can easily penetrate by paracellular transport to the systemic circulation. Finally, in recent decades, the colon has been postulated as an area of high interest for drug delivery. The physiological characteristics of the colon and the high residence times in this area facilitate absorption, especially for drugs that are degradable by intestinal enzymes. Furthermore, colonic administration allows the local treatment of certain pathologies such as inflammatory bowel diseases (IBD) or colorectal cancer [4,5,6].
Successful colonic administration requires protection of the drug against different pHs, enzymes, microorganisms, or peristaltic movements through the GIT [6,7]. Currently, capsules or tablets with pH-dependent, pressure-dependent, or time-modified release coatings are used for colonic delivery. Once in the colon, the drug should be released from the delivery system at specific rates and solubilized in the tissues, avoiding toxic effects and getting an optimum pharmacological response [6,8]. Unfortunately, there are great intra-individual and inter-individual variabilities due to the gastric and intestinal transit times, volumes of liquid through the GIT, diet, food–drug interactions, pathologies, gender, or age of patients that compromise the efficacy of the formulations [9].
Polysaccharides have been proposed as the main excipients for colonic formulations, as coating materials, matrices, hydrogel precursors, or prodrug ingredients [6,10,11]. Their physicochemical properties confer on them enzymatic and/or pH resistance. This allows polysaccharides to pass unaltered through the GIT, thus protecting the drug. Then, once in the colonic area, polysaccharides are decomposed by a microbiota composed of a huge number of microorganisms (1011 CFU/g fecal content), allowing the complete release of the drug [10,11]. Polysaccharides also have some industrial advantages, like their abundance and low price, biodegradability, and non-toxicity for the environment [11,12,13].
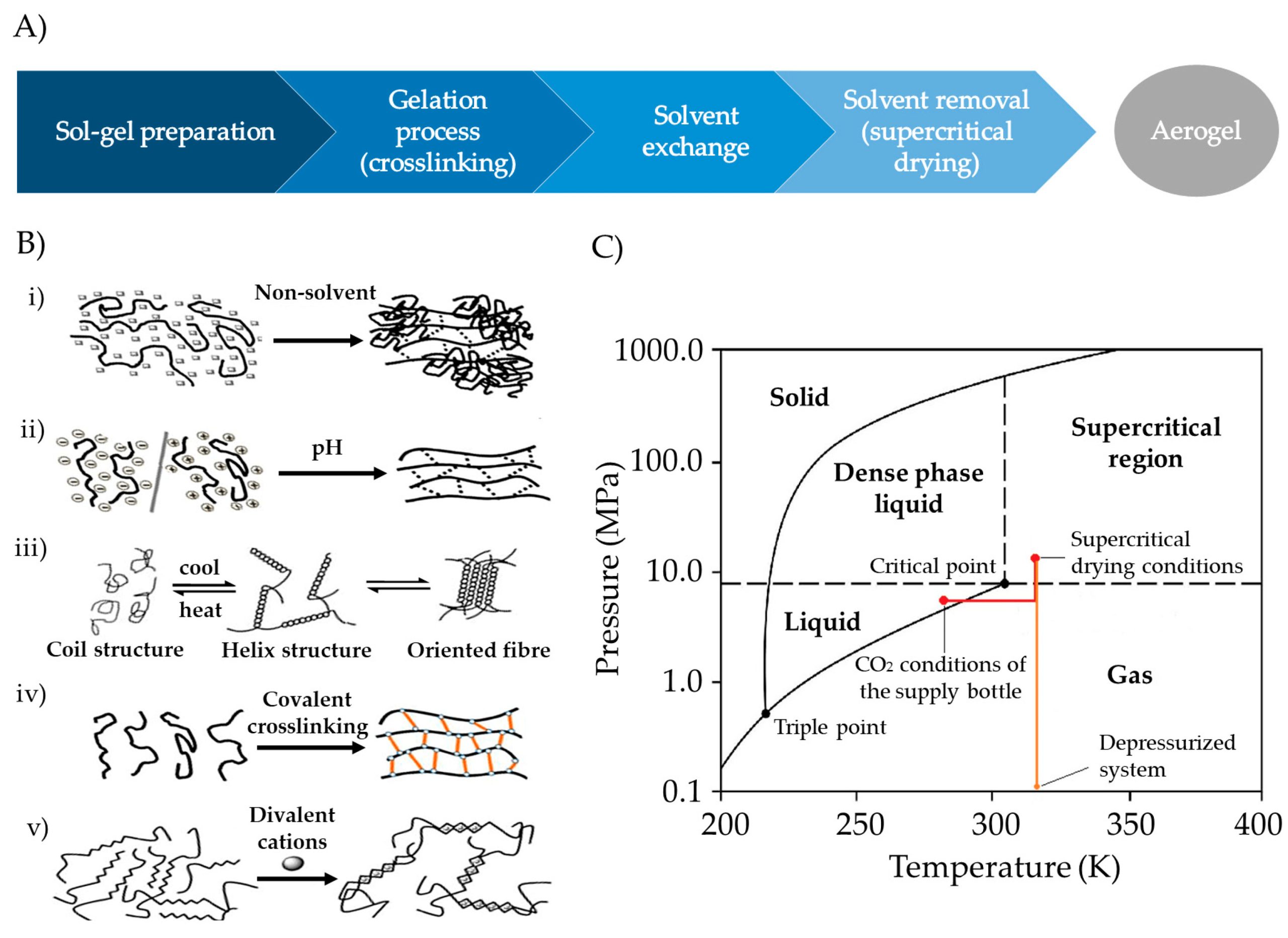
Hydrogels can be formed from polysaccharides by physical or chemical crosslinking, giving rise to three-dimensional systems of high porosity that can host and protect drugs and then release them in response to different stimuli [14,15,16]. Dry gels are advantageous over hydrogels in terms of drug stability as they are less susceptible to microbiological and chemical degradation [17]. Polysaccharide-based hydrogels can be dried using various methods, and their choice extraordinarily affects the structural properties of the resulting dry gel. Heat drying results in xerogels with significant structural shrinkage, and freeze-drying yields cryogels suitable for certain pharmaceutical applications like oral disintegrating formulations (e.g., ODTs) or scaffolds [18,19]. Supercritical fluid (SCF) drying produces dry gels with a preserved porous nanostructure (the so-called aerogels) and different formats and sizes depending on the chosen preparation technique [20].
Aerogels are dry solids with extremely low weight and bulk density (<0.2 g/m3), high porosity (>90%) predominantly in the mesoporous range (2–50 nm), and high specific surface area (>200 m2/g) [20,21]. They can be prepared from a wide range of inorganic and organic sources, including polysaccharides. The initial mesoporosity of the wet structures is preserved in the aerogels, which gives them a high surface area and excellent drug-loading capacity in the amorphous state [20]. A significant proportion of newly discovered New Molecular Entities (NME) fail to realize their full clinical potential, primarily because of their limited aqueous solubility, stability issues, and, in many instances, inadequate tissue targeting properties. The possibility of loading and stabilizing drugs in aerogels in an amorphous state opens new perspectives for the development of pharmaceutical formulations with poorly soluble NME. Its incorporation into mesoporous structures that remain in a metastable state would improve its dissolution rate, overcoming this problem [22].
A number of aerogel formulations for drug delivery have been recently investigated and proposed for different applications, such as pulmonary drug delivery [23], wound healing [24], or oral administration of drugs [20]. Various polysaccharides, such as alginate, pectin, or starch, among others, processed using various technologies such as emulsification or drip-gelation, have demonstrated their usefulness in producing aerogel particles that can incorporate drugs in their amorphous state. Furthermore, they exhibit modified drug release profiles, offering promising opportunities for enhancing drug absorption and stability [22,25,26].
The enzymatic- and/or pH-resistance of certain polysaccharide aerogels also makes them potential candidates for the development of colonic delivery dosage forms [6]. This approach is attractive for delivering biologics like peptides, given their reduced susceptibility to proteolytic degradation in the colon compared to other sections of the GIT. Additionally, the extended residence times and favorable pH levels in this region contribute to improved bioavailability [27,28]. However, the requirements of colonic formulations are higher than those of conventional oral dosage forms (burst release) [15]. The release of the drug must be specific and controlled in the colonic area. Therefore, the formulation must bypass the aforementioned adverse conditions of the GIT until reaching the colon and then release the drug at the appropriate rate to ensure that no toxic effects or problems of therapeutic inefficacy occur [29].
This review explores recent advances in porous polysaccharide-based formulations for oral drug delivery, providing a critical assessment of aerogels’ potential for colonic drug delivery for the first time. The sections of this article will cover an overview of the GIT characteristics with a special focus on the large intestine, the selection of suitable polysaccharides (alginate, chitosan, pectin, cellulose, starch, and glucomannan, among others) for colonic drug delivery, as well as the analysis of formulation strategies for aerogel design and prior art on aerogels with potential colonic application, including their preparation methods. Finally, alternative technological approaches for producing aerogels with appropriate properties for colonic drug delivery will be presented as future perspectives.
Excipients mentioned in this review beside others: Eudragit® S
Download the full review as PDF here: Aerogels as Carriers for Oral Administration of Drugs: An Approach towards Colonic Delivery
or read it here
Illanes-Bordomás, C.; Landin, M.; García-González, C.A. Aerogels as Carriers for Oral Administration of Drugs: An Approach towards Colonic Delivery. Pharmaceutics 2023, 15, 2639.
https://doi.org/10.3390/pharmaceutics15112639

