Unlock the Potential of Poorly Soluble Drugs with Apisolex™ Technology
Don’t let poor solubility affect your innovative drug projects

Approximately 60% of potential active pharmaceutical ingredients (APIs) under development, and more than 40% of those in reformulation, are poorly water soluble1. Solubility challenges present a substantial hurdle to the development of pharmaceuticals, impeding or stopping the advancement of promising drugs to market. There are several different approaches to overcome solubility challenges:
• Chemical modification — creating a prodrug of the API
• Physical processing — nanomilling to reduce particle size
• Excipients – formulating with cosolvents, lipids, surfactants, etc.
Increase API solubility by up to 50,000-fold with Apisolex Technology
Excipients have long played a key role in addressing solubility challenges in drug development. Due to their inherent versatility, they provide a tool to formulate poorly soluble APIs into products with improved bioavailability and therapeutic effects. However, as the number of poorly soluble APIs grows, existing excipients are still not able to solve the complex formulation challenges. Particularly in the parenteral space, there are few excipients acceptable to aid the formulation of novel APIs. The unique safety and chemical challenges of developing an excipient for parenteral applications have made commercialization a rare event over the last 30 years. Of those currently available in the market, some come with unpleasant patient side effects and do not address the solubility needs of highly crystalline and hydrophobic APIs.
With existing excipients falling short, developing a solution has been a key commitment of Lubrizol Life Science Health (LLS Health). In this guide, you will learn more about Apisolex™ technology, what makes it unique, and how it can help you drive your parenteral drug product innovation to success.
Unlike Apisolex, PEG-based excipients may produce significant side effects
including neuropathy and anaphylactic reactions
Benefits of Apisolex Technology
Innovation is inherent to the pharmaceutical industry. The Apisolex excipient provides a tool to improve solubility and offers drug developers, caregivers, and most importantly patients access to new and improved drug products.
Formulation Benefits of Apisolex Polymer
Safe, poly-amino acid based amphiphilic polymer which is a non-toxic, non-immunogenic, biocompatible, and biodegradable alternative to PEG
- Increases solubility of hydrophobic APIs by up to 50,000-fold, with amorphous and crystalline APIs.
- High drug loading (up to 40:100 API:solubilizer ratio).
- Forms stable, lyophilized drug product that reconstitutes in less than 30 seconds in saline.
- Patented technology enabling IP protection and lifecycle management/505(b)(2) formulations.
Processing Benefits of Apisolex Polymer
• Simple formulation techniques — solution mixing or oil-in-water emulsion formation — with >90% API recovery.
• Standard, scalable formulation techniques.
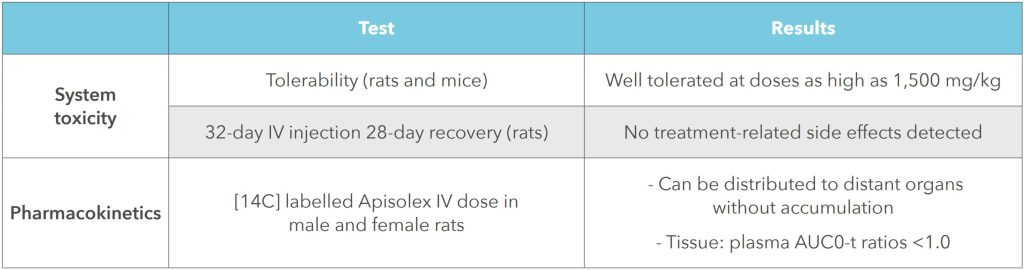
Safe for Parenteral Use
Apisolex is a GMP compliant polymeric excipient constituted of amino acid building blocks.
It’s biocompatible, biodegradable and tested for safety in parenteral projects:
Simplify Manufacture and Reduce Development Timelines
Apisolex technology has been designed to work with the simplest of formulation techniques, helping you streamline your manufacture and save precious time during drug development. The excipient and the hydrophobic API can be combined in an aqueous based solution followed by sterile filtration and lyophilization to produce a stable formulation that is readily resuspended in common diluents for administration. This technique forms micellular structures which encapsulate the API during lyophilization. In saline, the lyophilized drug product reconstitutes in less than 30 seconds.
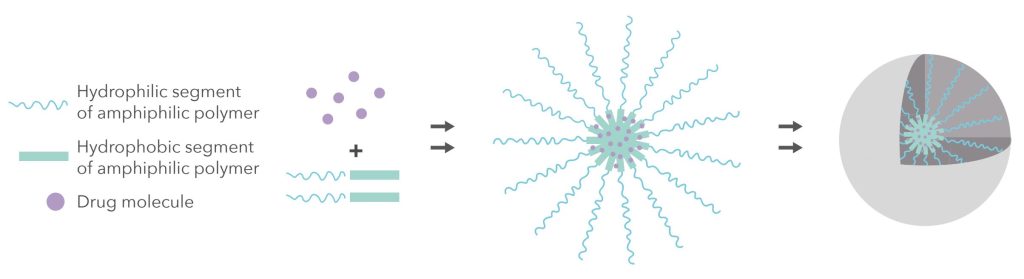
Innovative Design
Apisolex technology utilizes an amphiphilic multi block copolymer. This incorporates a hydrophilic poly(sarcosine) block and a second drug-encapsulating block comprised of a mixture of hydrophobic D- and L- poly (amino acids). As a naturally occurring amino acid, polymers of sarcosine offer an inert replacement to traditional water-soluble polymers such as PEG and Polyvinyl Alcohol (PVA).
The design features of the Apisolex polymer result in an innovative excipient that outperforms alternatives.
See the full brochure on “Apisolex™ Technology” here
(click the picture to download the brochure)
Source: Lubrizol brochure “Apisolex™ Technology”
Do you need more information or a sample of Apisolex™?