Development of a Carvedilol Oral Liquid Formulation for Paediatric Use
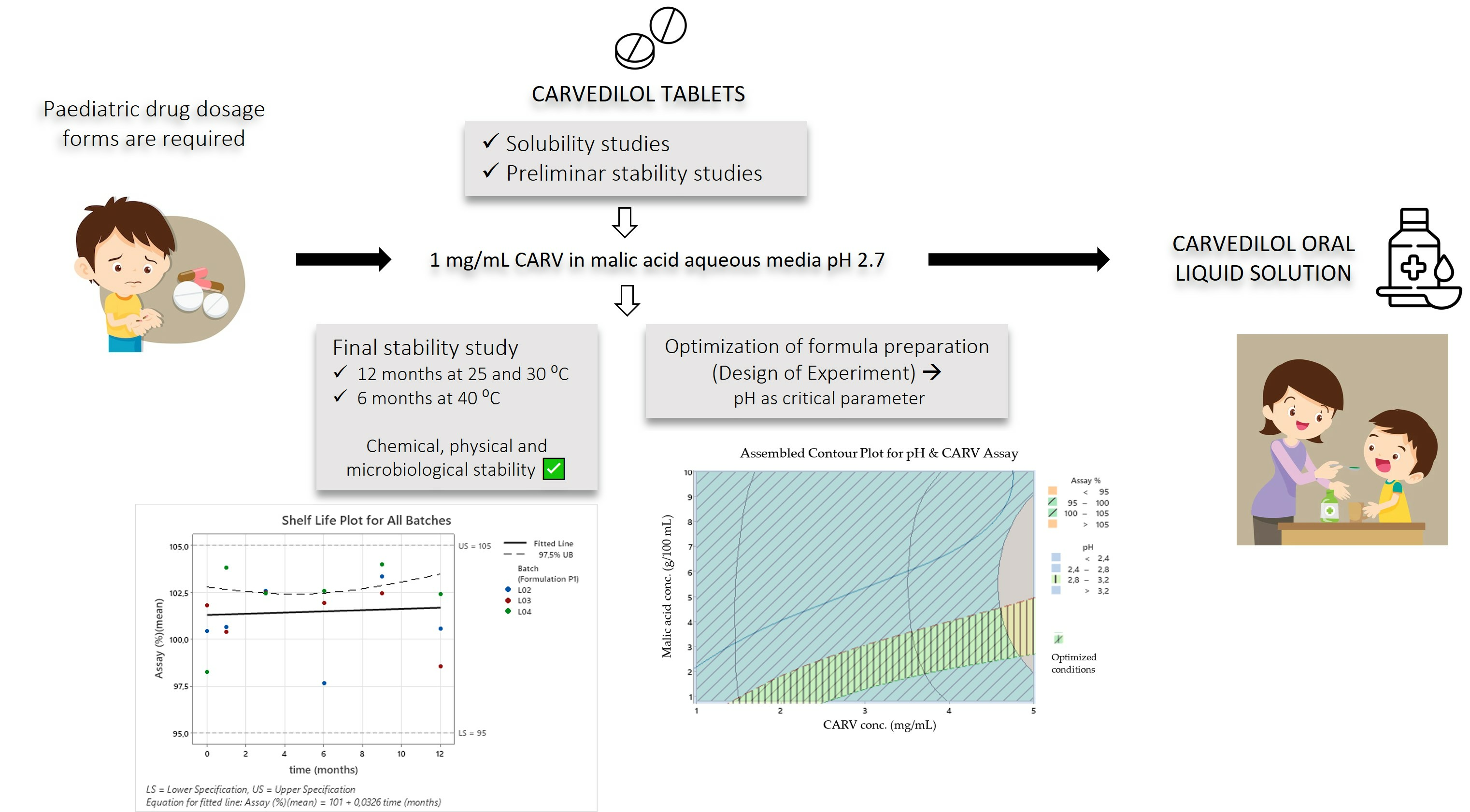
Carvedilol (CARV) is an ‘off-label’ β-blocker drug to treat cardiovascular diseases in children. Since CARV is nearly insoluble in water, only CARV solid forms are commercialized. Usually, CARV tablets are manipulated to prepare an extemporaneous liquid formulation for children in hospitals. We studied CARV to improve its aqueous solubility and develop an oral solution. In this study, we assessed the solubility and preliminary stability of CARV in different pH media. Using malic acid as a solubility enhancer had satisfactory results. We studied the chemical, physical, and microbiological stability of 1 mg/mL CARV–malic acid solution. A design of experiment (DoE) was used to optimize the CARV solution’s preparation parameters. A 1 mg/mL CARV solution containing malic acid was stable for up to 12 months at 25 °C and 30 °C and 6 months at 40 °C. An equation associating malic acid with CARV concentrations was obtained using DoE. Microbiological data showed that the use of methylparaben was not necessary for this period of time. We successfully developed an aqueous CARV solution suitable for paediatrics and proven to be stable over a 12-month period.
Download the full article as PDF here: Development of a Carvedilol Oral Liquid Formulation for Paediatric Use
or read it here
Pharmaceutical Development Chemicals
European Pharmacopoeia-grade (Ph. Eur.) carvedilol was kindly donated by Moehs (Barcelona, Spain). Ph. Eur.-grade malic acid-DL was purchased from FAGRON IBERICA (Barcelona, Spain). Other materials included dihydrogen potassium phosphate, sodium acetate, and citric acid monohydrate from Merk (Barcelona, Spain). We also obtained glacial acetic acid, hydrochloric acid, ortho-phosphoric acid, and sodium hydroxide from Panreac Applichem (Barcelona, Spain).
Chiclana-Rodríguez, B.; Garcia-Montoya, E.; Rouaz-El Hajoui, K.; Romero-Obon, M.; Nardi-Ricart, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; Pérez-Lozano, P. Development of a Carvedilol Oral Liquid Formulation for Paediatric Use. Pharmaceutics 2023, 15, 2283. https://doi.org/10.3390/pharmaceutics15092283
See the webinar:
“Fast Track development of Biphasic nano-dexamethasone Pellets using galenIQ™”, 12 October 2023:
Get more information & register here for free:


