Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types
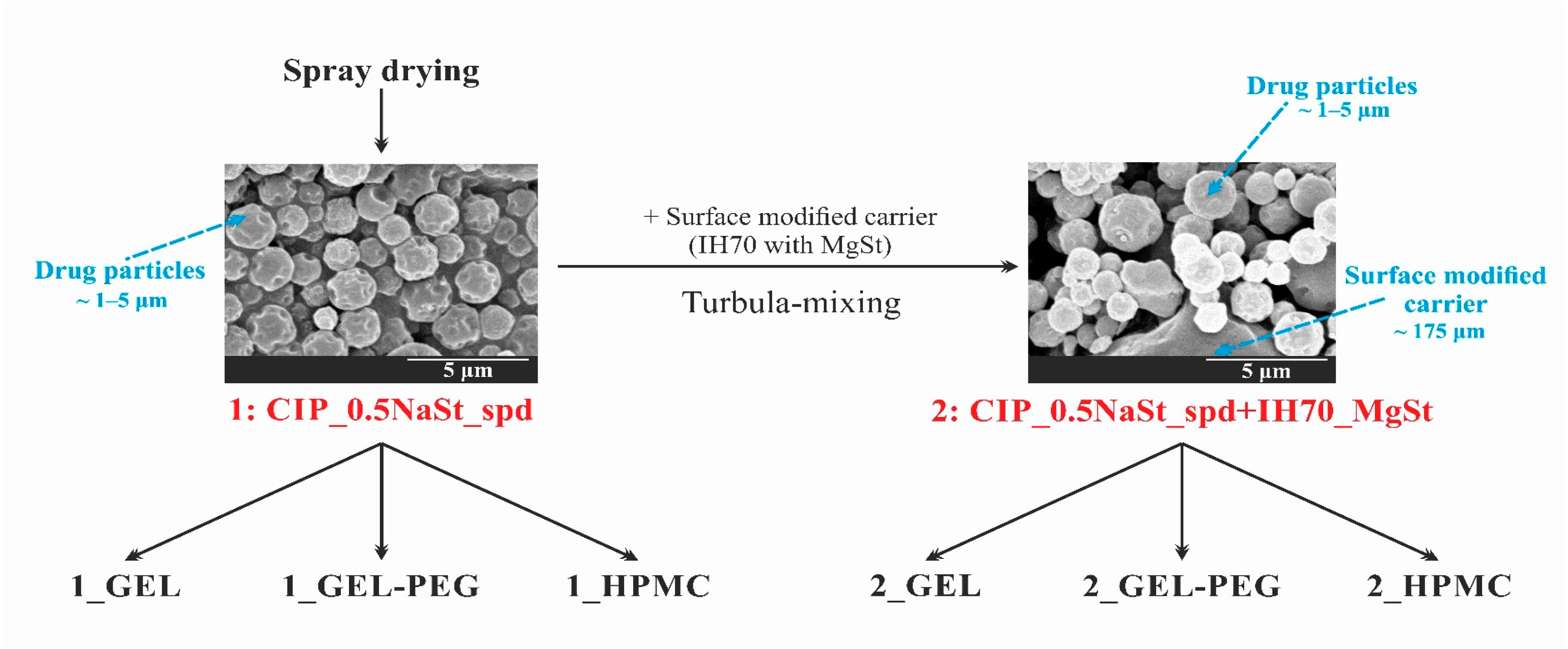
In the case of capsule-based dry powder inhalation systems (DPIs), the selection of the appropriate capsule is important. The use of gelatin, gelatin-PEG, and HPMC capsules has become widespread in marketed capsule-based DPIs. We aimed to perform a stability test according to the ICH guideline in the above-mentioned three capsule types. The results of the novel combined formulated microcomposite were more favorable than those of the carrier-free formulation for all capsule types. The use of HPMC capsules results in the greatest stability and thus the best in vitro aerodynamic results for both DPI powders after six months. This can be explained by the fact that the residual solvent content (RSC) of the capsules differs. Under the applied conditions the RSC of the HPMC capsule decreased the least and remained within the optimal range, thus becoming less fragmented, which was reflected in the RSC, structure and morphology of the particles, as well as in the in vitro aerodynamic results (there was a difference of approximately 10% in the lung deposition results). During pharmaceutical dosage form developments, emphasis should be placed in the case of DPIs on determining which capsule type will be used for specific formulations.
Download the full article here: Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types
or continue reading here: Benke, E.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics 2021, 13, 689. https://doi.org/10.3390/pharmaceutics13050689
Materials
Micronized ciprofloxacin hydrochloride (μCIP) (D (0.5): 5.09 μm) as a fluoroquinolone antibiotic active ingredient was applied and donated by Teva Pharmaceutical Works Ltd. (Debrecen, Hungary). Lactose monohydrate, Inhalac® 70 (IH 70) (D (0.5): 215.00 μm) was gifted by MEGGLE Group (Wasserburg, Germany) and utilized as a carrier. Magnesium stearate (MgSt) (D (0.5): 6.92 μm) was used to treat the surface of IH 70 [32], which was supplied by Sigma-Aldrich (Budapest, Hungary). Sodium stearate (NaSt) (Alfa Aesar, Heysham, United Kingdom) was used as an excipient in the co-spray-drying process. The Coni-Snap® hard GEL (Capsugel®/Lonza Pharma & Biotech, Basel, Switzerland), Ezeefit™ GEL-PEG (ACG-Associated Capsules Pvt. Ltd., Mumbai, India) and Ezeeflo™ HPMC (ACG-Associated Capsules Pvt. Ltd., Mumbai, India) capsules were used to store DPI formulations during the stability test.
Conclusions
In this study we introduced the importance of final formulation-development by studying the effect of capsule types on the stability and aerodynamic properties of DPI. The same formulation have different stability and thus aerodynamic properties in different DPI capsule types. The RSC and light microscopic results of the DPI capsules supported the claim that GEL and GEL-PEG-type capsules begin to break when the RSC falls below the optimal range. Due to their fragmentation, the resulting holes became irregularly shaped and large. Although more formulations came out of these larger, irregularly shaped holes, resulting in increased EF values, the deaggregation of the particles was less efficient, which in turn reduced FPF values. However, HPMC capsules retained their elasticity after 6 months, pieces of the capsule wall did not break during punching, and the holes remained in regular shape. RSC and XRPD analysis confirmed, and the SEM images also showed that DPI powders stored in GEL and GEL-PEG capsules formed irregularly shaped particles during the stability study due to the onset of recrystallization (it is assumed that moisture was transferred to DPI powders). The altered habit was aerodynamically disadvantageous, which may have been one of the reasons for the decrease in FPF values. The morphological change was least observed with the formulations stored in HPMC capsules, and FPF values decreased to a lesser extent. Overall, initial, almost identical aerosolization values after 6 months were the most favorable for HPMC capsules for both investigated DPI formulations. This was probably due to the RSC of the capsules, the size and shape of the perforated area, and the altered habit of the DPI powder. The results of the novel combined formulated composite were more favorable after the stability test than those of the carrier-free formulation for all DPI capsule types.
Thus, it may be worthwhile focusing on testing DPI formulations in different capsules during pulmonary dosage form development, as the same formulation may have different stability and thus aerodynamic properties in different DPI capsule types. The prepared DPI formulation of a carrier-free and novel combined carrier-based systems using CIP could present an effective new possibility in the therapy of lung diseases (direct and indirect treatment of pathophysiological processes such as cystic fibrosis and chronic bronchitis) instead of the per os applied antibiotic formulation.

