Customizable orodispersible films: Inkjet printing and data matrix encoding for personalized hydrocortisone dosing
Abstract
The aim of this study was to exploit the versatility of inkjet printing to develop flexible doses of drug-loaded orodispersible films that encoded information in a data matrix pattern, and to introduce a specialised data matrix-generator software specifically focused on the healthcare sector. Pharma-inks (drug-loaded inks) containing hydrocortisone (HC) were developed and characterised based on their rheological properties and drug content. Different strategies were investigated to improve HC solubility: formation of β-cyclodextrin complexes, Soluplus® based micelles, and the use of co-solvent systems. The software automatically adapted the data matrix size and identified the number of layers for printing. HC content deposited in each film layer was measured, and it was found that the proportion of co-solvent used directly affected the drug solubility and simultaneously played a role in the modification of the viscosity and surface tension of the inks. The formation of β-cyclodextrin complexes improved the drug quantity deposited in each layer. On the contrary, micelle-based inks were not suitable for printing. Orodispersible films containing flexible and low doses of personalised HC were successfully prepared, and the development of a code generator software oriented to medical use provided an additional, innovative, and revolutionary advantage to personalised medicine safety and accessibility.
Introduction
Conventional therapies follow a “one size fits all” approach, producing medicines with fixed doses for the entire population. While suitable for many, this approach poses challenges for drugs with a narrow therapeutic index (Wening and Breitkreutz, 2011) or highly variable pharmacokinetic and/or pharmacodynamic profiles (Edinger et al., 2018, Vuddanda et al., 2018, El-Maouche et al., 2017, Abdel Jalil et al., 2021). The same strength administered to several patients may cause varied pharmacological responses due to interindividual factors, leading to under or over-dosing (Turner et al., 2015). Such undesirable therapeutic outcomes are more notable in the paediatric population because of the lack of child-appropriate formulations. It is common practice to modify dosage forms designed for adults before administering to children, by preparing a suitable unlicensed medicine or by manipulating dosage forms at the point-of-care (Khan et al., 2022).
Hydrocortisone (HC) is the recommended glucocorticoid for treating adrenal insufficiency in childhood and puberty (deficiency of cortisol) (Nisticò et al., 2022). Cortisol production follows a circadian rhythm (Mohd Azmi et al., 2021), peaking in the morning and gradually decreasing throughout the day, reaching minimal levels at midnight. It is vital for patients to mimic this physiological pattern of cortisol production in their hormone replacement therapy to avoid decompensations (Chan and Debono, 2010) such as metabolic disorders, bone or weight loss, and cardiovascular disorders (Husebye et al., 2021). However, the replication of this pattern faces challenges due to the lack of versatility in the current manufacturing technologies. In clinical practice, the appropriate HC dose (8–10 mg/m2/day) is calculated based on the body surface area and is administered between 2–4 times a day (El-Maouche et al., 2017).
Up to now, hydrocortisone medicines specific to the paediatric population are scarce. While Plenadren® modified-release tablets are approved by the European Medicines Agency (EMA) in certain doses, safety and efficacy data for paediatric use is still limited (“Plenadren Product information. European Medicines Agency (EMA),” n.d.). Hydrocortisone modified-release capsules containing doses of 5, 10, or 20 mg (Efmody®) are approved by the EMA but are only indicated for adolescents > 12 years old and adults (“Efmody Product information. European Medicines Agency (EMA), 2023,” n.d.). The recent Food and Drug Administration (FDA) and EMA approval of Alkindi® marks the first paediatric medicine for this disorder (birth to <18 years), available in capsules with hydrocortisone granules ranging from 0.5 mg to 5 mg (“Alkindi Product information. European Medicines Agency (EMA), 2023,” n.d.). However, availability issues exist in certain European countries like Spain due to observed side effects when discontinuing conventional hydrocortisone treatment (“Alkindi Product information. European Medicines Agency (EMA), 2023,” n.d.). As a result, HC formulations containing the specific dose are prepared manually at the dispensing point, and marketed tablets are crushed or HC suspensions are made in hospital pharmacies to comply with specific patient needs (Whitaker et al., 2015). However, pharmaceutical compounding is time consuming, resource-intensive, prone to dosing errors, and is an inflexible approach to meet continuous dose changes (Watson et al., 2021).
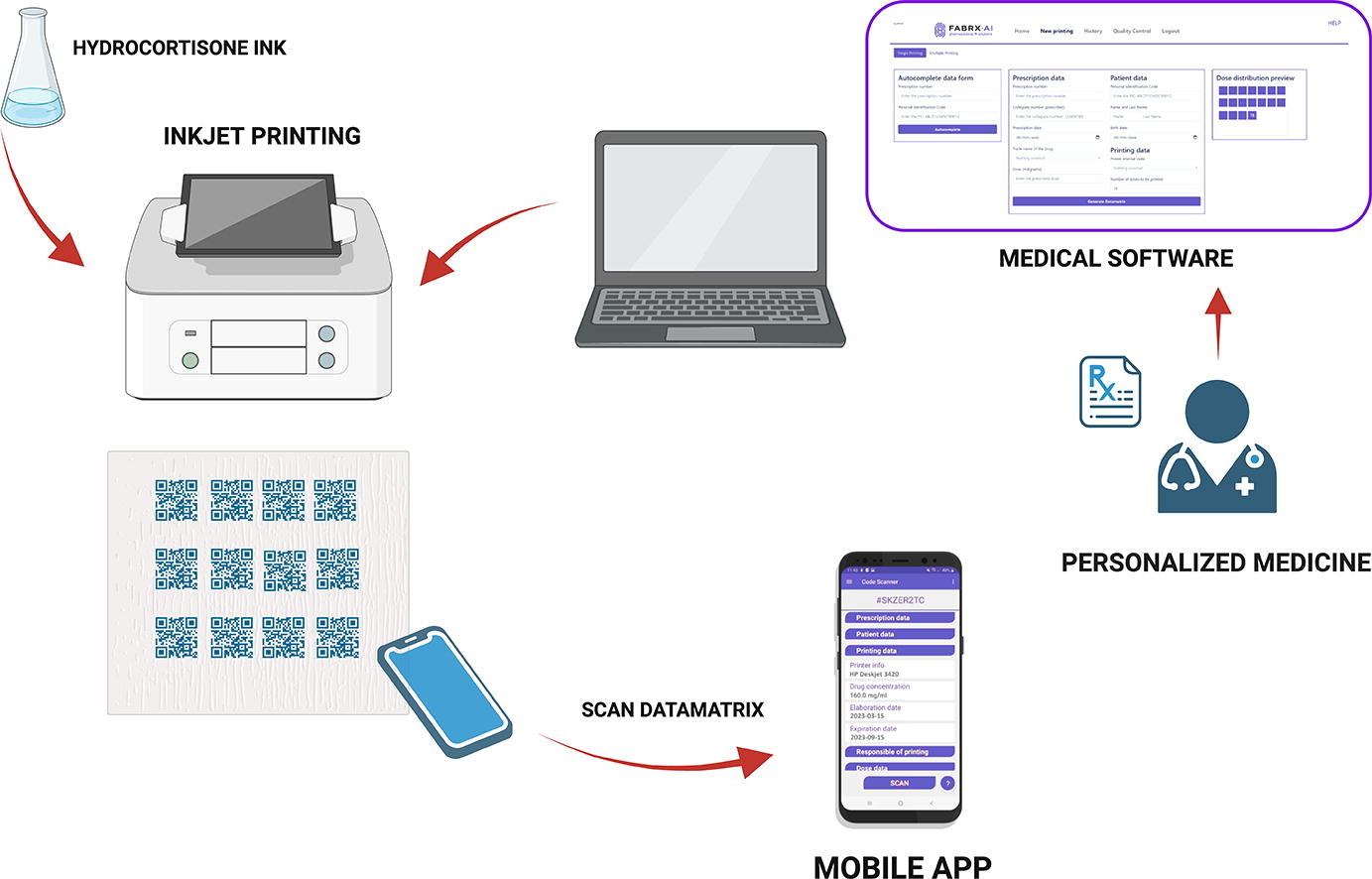
In the last decade, various additive manufacturing technologies have been explored to ensure a more personalized patient treatment approach (Cui et al., 2021, Daly et al., 2015, Nadagouda et al., 2020, Seoane-Viaño et al., 2021, Vaz and Kumar, 2021). Inkjet printing is gaining attention in the development of tailored oral dosage forms (Boehm et al., 2014, Carou-Senra et al., 2023, Chou et al., 2021, Kiefer et al., 2021, Öblom et al., 2020, Sandler et al., 2011). Traditional desktop inkjet printers have been successfully adapted to print a vast number of drugs such as caffeine and indomethacin on different substrates (Alomari et al., 2018, Arshad et al., 2020, Genina et al., 2013, Genina et al., 2012, Kiefer et al., 2021, Wickström et al., 2015). This material jetting technology involves the deposition of a controlled droplet pattern of pharma-ink (drug-loaded ink) on a substrate (Daly et al., 2015, Singh et al., 2010). This technology can be classified according to the mechanism of droplet generation. Thermal inkjet printing uses micro-resistors in direct contact with the ink, rapidly heating up to form a vaporization-induced bubble that expands, ejecting fluid through the nozzle to create a droplet (Meléndez et al., 2008). In piezoelectric inkjet printing, each nozzle is surrounded by a piezoelectric element that, when subjected to an electrical current, mechanically deforms the ink, generating pressure waves that expel droplets through the nozzle (Azizi Machekposhti et al., 2019). Inkjet printing has demonstrated success in various dosage forms, such as orodispersible or bioadhesive films (Kiefer et al., 2021, Varan et al., 2017, Vuddanda et al., 2018), transdermal microneedles (Boehm et al., 2013), contact lenses (Pollard et al., 2023), drug-loaded stents (Scoutaris et al., 2016) and even direct application onto nails (Pollard et al., 2022).
The versatility and precision of inkjet printing technology allows the design of unique digital patterns on substrates, introducing novel dosage forms (data-enriched edible pharmaceuticals – DEEPs), with information easily readable by smartphones (Chao et al., 2022, Chao et al., 2021, Edinger et al., 2018, Öblom et al., 2020). The required dose is printed as a QR or data matrix code, providing encoded information such as drug name, dose, patient details, prescribing instructions, side effects, expiry date, manufacturer identification and the batch number to patients and/or healthcare professionals (Handa et al., 2023). The use of QR codes and smart devices has demonstrated improvements in tracking, safety, and adherence to prescribed dosage schedules, leading to reduced visits to health professionals (Chao et al., 2021, Edinger et al., 2018). Although studies have explored QR code printing on films using free online QR generators, the development of healthcare-specific software remains unexplored (Chao et al., 2021, Edinger et al., 2018). A specialised software would ensure the tracking and traceability of the drug product directly to the patient, presenting a significant opportunity to utilize inkjet printing to prepare data-enriched dosage forms, improving treatment safety and efficacy (Nørfeldt et al., 2019, Raijada et al., 2021).
The aim of this study was to evaluate the combination of inkjet printing and a data matrix code generator software specifically made for the healthcare sector, to produce data matrix films loaded with HC as the model drug. Various HC-loaded inks, incorporating different excipients, were developed to optimize inkjet printing performance. Inclusion complexes and micelle formation were evaluated to improve poor drug solubility within the pharma-ink, since they are available pharmaceutical strategies to overcome solubility issues of poorly water-soluble drugs (Rodriguez-Aller et al., 2015). The pharma-inks were printed on edible substrates using conventional desktop printers, following a data matrix pattern generated by the developed software. The study varied the number of printed layers and data matrix size to achieve personalized doses, quantifying the total HC printed in different layers.
Download the full article as PDF here Customizable orodispersible films
or read it here
Materials
Hydrocortisone base (MW 362.46 g/mol, Acofarma, Barcelona, Spain); 1,2-propylene glycol (Scharlau, Barcelona, Spain); MilliQ® water; Methanol (Merck, Darmstadt, Germany); Ethanol (VWR International, Radnor, USA); E-122 Red colorant (Guinama, Valencia, Spain); Bright blue colorant (Guinama, Valencia, Spain); β-Cyclodextrin (β-CD; Nihon Shokuhin Kako Co. Ltd, Tokyo, Japan); Soluplus® (polyvinyl caprolactam polyvinyl acetate-polyethylene glycol grafted copolymer, BASF, Ludwigshafen, Germany); SensiJet® (Sensient, Milwaukee, USA); Potato starch edible paper (0.3 mm thickness; Decoración Dulce, Madrid, Spain); Rice edible paper (0.4 mm thickness; Decoración Dulce, Madrid, Spain).
Lucía Rodríguez-Pombo, Paola Carou-Senra, Erea Rodríguez-Martínez, Patricija Januskaite, Carlos Rial, Paulo Félix, Carmen Alvarez-Lorenzo, Abdul W. Basit, Alvaro Goyanes, Customizable orodispersible films: Inkjet printing and data matrix encoding for personalized hydrocortisone dosing, International Journal of Pharmaceutics, 2024, 124005, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124005.
See our next webinar and register here:


