The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems
Abstract
Self-emulsification is considered a formulation technique that has proven capacity to improve oral drug delivery of poorly soluble drugs by advancing both solubility and bioavailability. The capacity of these formulations to produce emulsions after moderate agitation and dilution by means of water phase addition provides a simplified method to improve delivery of lipophilic drugs, where prolonged drug dissolution in the aqueous environment of the gastro-intestinal (GI) tract is known as the rate-limiting step rendering decreased drug absorption. Additionally, spontaneous emulsification has been reported as an innovative topical drug delivery system that enables successful crossing of mucus membranes as well as skin. The ease of formulation generated by the spontaneous emulsification technique itself is intriguing due to the simplified production procedure and unlimited upscaling possibilities.
However, spontaneous emulsification depends solely on selecting excipients that complement each other in order to create a vehicle aimed at optimizing drug delivery. If excipients are not compatible or unable to spontaneously transpire into emulsions once exposed to mild agitation, no self-emulsification will be achieved. Therefore, the generalized view of excipients as inert bystanders facilitating delivery of an active compound cannot be accepted when selecting excipients needed to produce self-emulsifying drug delivery systems (SEDDSs). Hence, this review describes the excipients needed to generate dermal SEDDSs as well as self-double-emulsifying drug delivery systems (SDEDDSs); how to consider combinations that complement the incorporated drug(s); and an overview of using natural excipients as thickening agents and skin penetration enhancers.
Introduction
Self-emulsification is considered a formulation technique that has proven capacity to improve oral drug delivery of poorly water-soluble drugs by advancing both the solubility and bioavailability of these active compounds [1]. The capacity of self-emulsifying drug delivery systems (SEDDSs) to produce emulsions after exposure to moderate agitation and dilution by means of water phase addition provides a simplified method to improve delivery of lipophilic drugs, where prolonged drug dissolution in the aqueous environment of the gastro-intestinal (GI) tract is known as the rate-limiting step, influencing drug absorption [1,2]. Additionally, spontaneous emulsification has furthermore been reported as an innovative topical drug delivery system that enables successful crossing of mucus membranes as well as skin [3,4,5].
The ease of formulation generated by the spontaneous emulsification technique itself is intriguing due to the simplified production procedure and unlimited upscaling possibilities [1,6]. However, spontaneous emulsification depends solely on selecting excipients that complement each other in order to create a vehicle aimed at optimizing drug delivery [1,5,6,7]. If excipients are not compatible or able to trigger the spontaneous emulsification process once exposed to mild agitation, no self-emulsification will be achieved. Therefore, the generalized view of excipients as inert bystanders facilitating delivery of an active compound cannot be accepted when selecting excipients needed to produce self-emulsifying drug delivery systems (SEDDSs) [1,8]. Hence, this review describes the excipients needed to generate more specifically dermal SEDDSs as well as self-double-emulsifying drug delivery systems (SDEDDSs), how to consider combinations that complement the incorporated drug(s), and an overview of using natural excipients as potential preservatives and skin penetration enhancers.
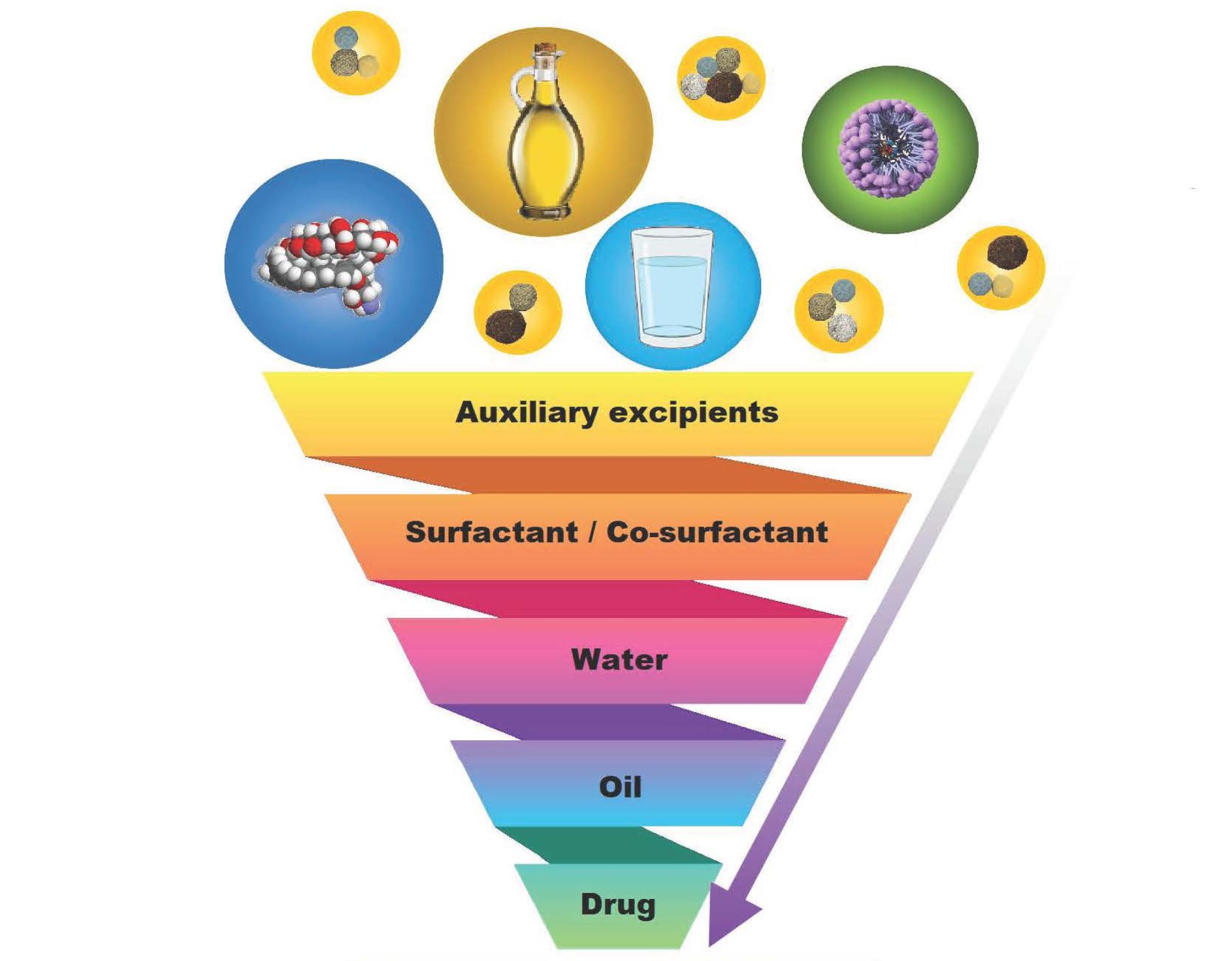
It has been established that self-emulsification depends on the chosen lipid/surfactant combination, surfactant concentration, as well as the ratio of lipid to surfactant [9]. Findings have also confirmed that only extremely precise excipient combinations can lead to the formation of spontaneous emulsions [1,9,10]. Hence, after selecting drug(s) considered for the incorporation into SEDDSs or SDEDDSs, the screening of excipients is significant in terms of compatibility, solubility, and stability [1,9]. However, when systems with multiple excipients are designed, the evaluated excipients should produce overall solubilization instead of only exhibiting sufficient solubility of a single excipient that forms part of the formulation [1]. Therefore, the included surfactant, co-surfactant, and oil phase(s) can be of a synthetic, semi-synthetic, or natural origin as long as toxicity and tissue irritancy are considered during excipient selection [1]. Importantly, excipients are generally selected for oral SEDDSs based on their (1) capacity to attain maximum drug loading; (2) ability to ensure maximum drug absorption as influenced by sufficient duration of self-emulsification, together with minimized droplet size; (3) limiting fluctuation of droplet size influenced by the pH of the aqueous medium as well as electrolyte concentration; and (4) limiting degradation of the administered drugs in the physiological environment [1,2,9]. On the other hand, excipients selected for dermal drug delivery must be considered based on their (1) capacity to attain maximum drug loading, but to a lesser degree when compared to drugs destined for oral absorption since dermal application allows delivery of higher drug concentrations directly to the affected site; (2) ability to ensure maximum dermal drug diffusion by including excipients that facilitate diffusion by limiting trans epidermal water loss (TEWL), through hydration (water), and/or humectant inclusion; (3) limiting droplet size fluctuation and maintain a decreased droplet size to improve dermal drug delivery as achieved by selecting surface active agents capable of providing sufficient stabilization without reaching toxic surfactant concentrations; and (4) including excipients such as skin penetration enhancers in order to achieve dermal lipid disruption required to facilitate drug diffusion into underlying skin layers [5,11,12,13,14].
Considering the numerous factors mentioned, when selecting excipients for dermal SEDDSs compared to oral SEDDSs, one can also regard these same factors if aiming to create dermal SDEDDs instead of SEDDSs. Dermal SDEDDSs provide the option of including even more active ingredients in combination as these multiple emulsions can offer more options for solubilization of numerous drugs in a single formulation compared to SEDDSs that are traditionally limited to a water and oil phase supported by the surfactant phase [15]. It has been reported, for example, that incorporating quercetin into an SDEDDS improved skin permeation compared to other dermal drug delivery systems [11]. Moreover, literature concluded that a rutin-loaded non-aqueous SDEDDS exhibited a sustained release profile of up to 12 h [12]. Therefore, studies have clearly indicated the advantages of utilizing SDEDDSs as multiple-emulsion dermal drug delivery systems if approached correctly. However, only a limited literature about dermal SEDDSs and SDEDDSs has already been published, which contributes to the lack of information when aiming to select excipients during the development of these unique systems. Table 1 lists recent publications related to the successful formulation of dermal spontaneous emulsions.
Download the full article as PDF here The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems
or read it here
Excipients mentioned in the paper beside others: Tween 20, Tween 80, Brij
van Staden, D.; Haynes, R.K.; Viljoen, J.M. The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems. Pharmaceutics 2023, 15, 1293. https://doi.org/10.3390/pharmaceutics15041293
Read more one Excipients for Parenterals here:


