Disruptive Materials – Pharma – presents a unique carrier for amorphous stabilization of poorly soluble drugs
Located right in between Uppsala University, the Swedish Medical Products Agency and Uppsala University Hospital, the company is well positioned to exploit its pioneering discovery of a unique mesoporous magnesium carbonate (MMC) material.  Up until breakthrough discovery by the group of professor Maria Strømme in 2012, commercialized by Disruptive Materials AB in 2013, nobody had been able to produce an amorphous and anhydrous mesoporous magnesium carbonate with such high surface area and pore volume. The discovery attracted a considerable media attention around the globe. The company didn’t rest on their successes and the Pharma team, headed by Dr. Peter Åsberg, continued the development of MMC into a reliable drug delivery system at a high pace.
Up until breakthrough discovery by the group of professor Maria Strømme in 2012, commercialized by Disruptive Materials AB in 2013, nobody had been able to produce an amorphous and anhydrous mesoporous magnesium carbonate with such high surface area and pore volume. The discovery attracted a considerable media attention around the globe. The company didn’t rest on their successes and the Pharma team, headed by Dr. Peter Åsberg, continued the development of MMC into a reliable drug delivery system at a high pace.
Pharmaceutical development team
 Disruptive Materials – Pharma – (DM Pharma) continued development of the original MMC platform into a novel drug delivery platform, the pharmaceutical grade mesoporous magnesium carbonate (Pharma-MMC). The pharmaceutical development team, headed by Dr Tuulikki Lindmark and Dr Jonas Fagerberg, at Disruptive Materials – Pharma – has in recent years developed the Pharma-MMC drug delivery system and successfully stabilized problematic, active pharmaceutical ingredients (API’s).
Disruptive Materials – Pharma – (DM Pharma) continued development of the original MMC platform into a novel drug delivery platform, the pharmaceutical grade mesoporous magnesium carbonate (Pharma-MMC). The pharmaceutical development team, headed by Dr Tuulikki Lindmark and Dr Jonas Fagerberg, at Disruptive Materials – Pharma – has in recent years developed the Pharma-MMC drug delivery system and successfully stabilized problematic, active pharmaceutical ingredients (API’s).
Amorphous stabilization
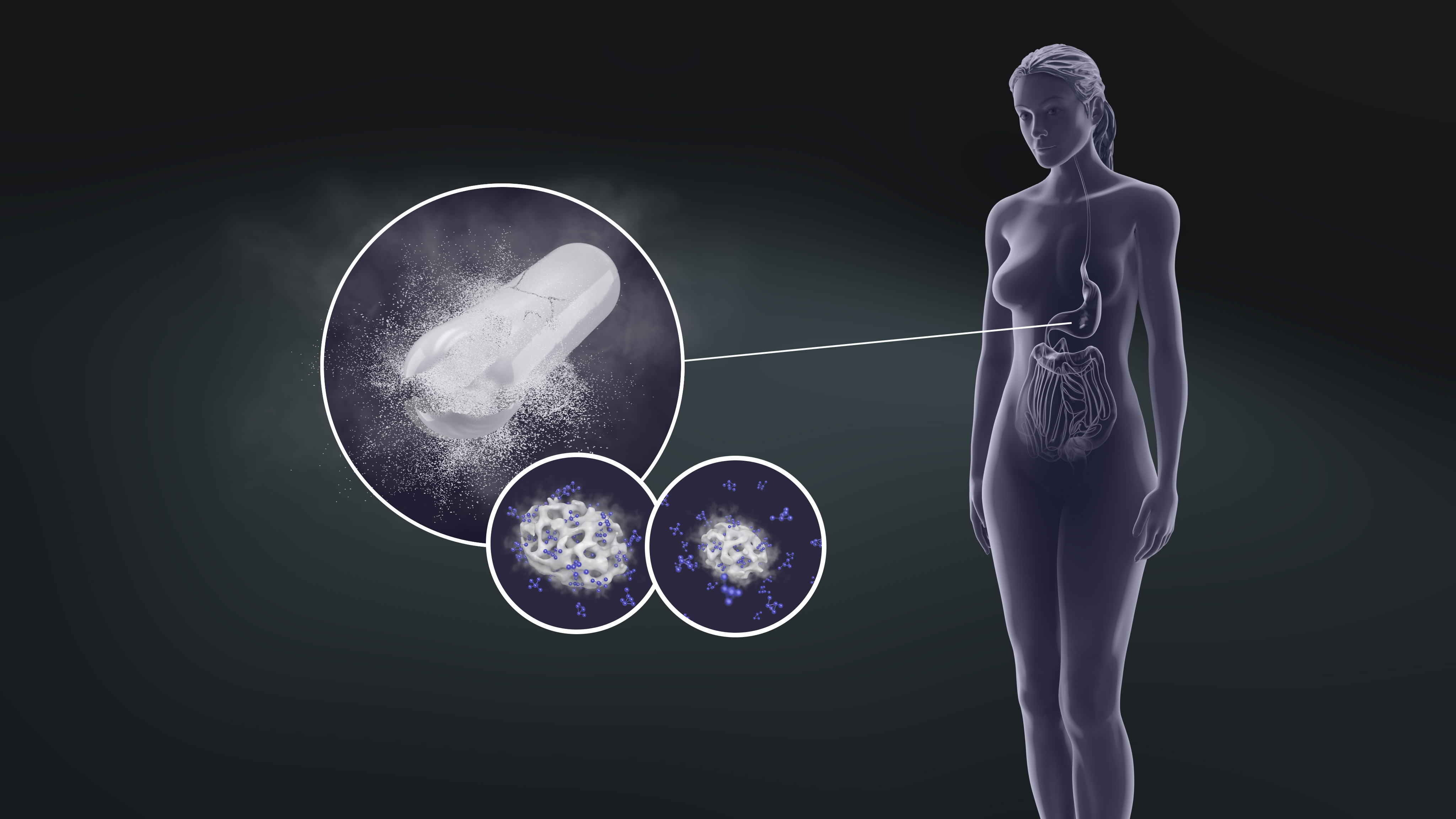
 It is a well-known fact that the dissolution rate is higher for the amorphous form of a poorly soluble drug than from its various crystal forms. However, the amorphous state is inherently unstable and tends to recrystallize back into the poorly soluble form. Accordingly, amorphous API’s as such are not feasible for the development of stable and high-quality oral drug products. Various approaches have been proposed for the stabilization of amorphous drugs. For example, hot melt extrusion and spray-drying. DM Pharma has now entered that scene with its proprietary Pharma grade mesoporous magnesium carbonate (Pharma-MMC) drug delivery platform and is featuring high drug loads and excellent stability. The team is now working with process optimization and to scale it up and then go the distance, to the market says Peter Åsberg.
It is a well-known fact that the dissolution rate is higher for the amorphous form of a poorly soluble drug than from its various crystal forms. However, the amorphous state is inherently unstable and tends to recrystallize back into the poorly soluble form. Accordingly, amorphous API’s as such are not feasible for the development of stable and high-quality oral drug products. Various approaches have been proposed for the stabilization of amorphous drugs. For example, hot melt extrusion and spray-drying. DM Pharma has now entered that scene with its proprietary Pharma grade mesoporous magnesium carbonate (Pharma-MMC) drug delivery platform and is featuring high drug loads and excellent stability. The team is now working with process optimization and to scale it up and then go the distance, to the market says Peter Åsberg.
Pharma-MMC may offer high drug loads and several other advantages
 Pharma-MMC is a safe and soluble drug carrier that can stabilize an active pharmaceutical ingredient (API) in its amorphous state. But Pharma-MMC may also offer another advantage compared to other solubilization technologies, such as cyclodextrins and liposomes among some. Using such other technologies may result in a rather low drug/excipient ratio, compelling an unfortunate choice between dosage strength and dosage size. Pharma-MMC on the other hand offer drug loads between 20-50% w/w and with such high drug load there is no contradiction between a high dose and patient compliance, by providing a tablet or capsule that is easy to swallow. Scientists at DM Pharma are now pushing forward, studying Pharma-MMC drug delivery characteristics and in vivo performance of selected API’s. At the same time we are also working on the scale-up of the Pharma-MMC manufacturing (lead by Dr Ingrid Ajaxon and Dr Peter Åsberg).
Pharma-MMC is a safe and soluble drug carrier that can stabilize an active pharmaceutical ingredient (API) in its amorphous state. But Pharma-MMC may also offer another advantage compared to other solubilization technologies, such as cyclodextrins and liposomes among some. Using such other technologies may result in a rather low drug/excipient ratio, compelling an unfortunate choice between dosage strength and dosage size. Pharma-MMC on the other hand offer drug loads between 20-50% w/w and with such high drug load there is no contradiction between a high dose and patient compliance, by providing a tablet or capsule that is easy to swallow. Scientists at DM Pharma are now pushing forward, studying Pharma-MMC drug delivery characteristics and in vivo performance of selected API’s. At the same time we are also working on the scale-up of the Pharma-MMC manufacturing (lead by Dr Ingrid Ajaxon and Dr Peter Åsberg).
It’s all about the products…
DM Pharma’s goal is to develop selected drug products based on a Pharma-MMC and API formulation within a market niche facilitated by our proprietary drug delivery system followed by targeted development towards the pharmaceutical industry. At the end of the day, it’s all about unmet medical needs says Peter Åsberg. If a highly effective but poorly soluble drug can make it through development and onto the market, it can mean a lot to a patient. Our goal is therefore to use Pharma-MMC unique features to bring several successful products to the market and truly make a difference. DM Pharma follows its business plan with primary goal to develop novel drug products, targeted for the pharmaceutical industry and the market encompassing the same. Pharma-MMC is used to create amorphous solid dispersions of APIs which are physically stable over time. Pharma-MMC also provides a great opportunity particularly for drugs with poor aqueous solubility, thus facilitating its dissolution and the body’s ability to absorb drugs in the GI tract. Disruptive Materials Pharma currently have multiple potential drug product candidates in development and patent applications on file.

Illustration of capsule containing an API loaded into Pharma-MMC that rapidly dissolves and releases API once it reaches the stomach. Click on the picture or here to see the video
INTERESTED TO HEAR MORE ABOUT PHARMA-MMC? CLICK HERE
 Peter Åsberg, PhD
Peter Åsberg, PhD
Sr Vice President – Pharma
Disruptive Materials AB
[email protected]
Cell: +46 70 949 17 21
Uppsala Science Park
SE-75183 Uppsala, Sweden

