A case study on distributed manufacturing of 3D printed medicines
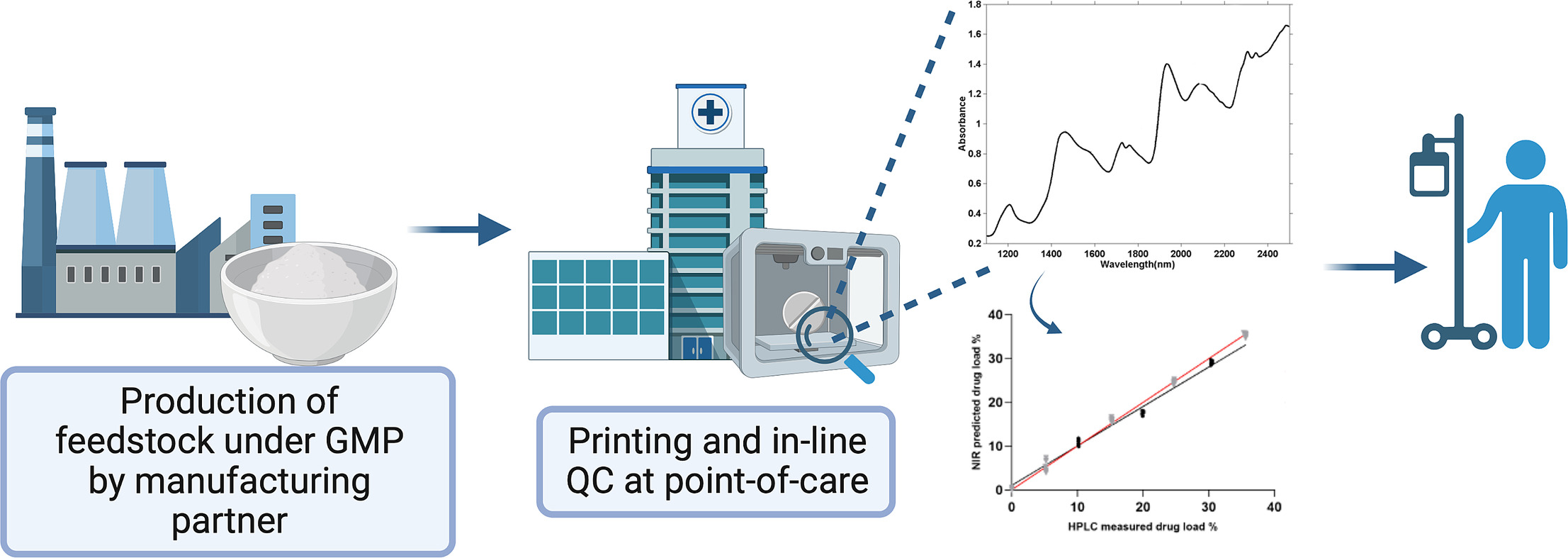
Pharmaceutical 3D printing (3DP) is one of the emerging enabling technologies of personalised medicines as it affords the ability to fabricate highly versatile dosage forms. In the past 2 years, national medicines regulatory authorities have held consultations with external stakeholders to adapt regulatory frameworks to embrace point-of-care manufacturing. The proposed concept of distributed manufacturing (DM) involves the provision of raw materials prepared by pharmaceutical companies to DM sites for manufacturing into the final medicine. In this study, we examine the feasibility of this model, with respect to both manufacturing and quality control. Efavirenz-loaded granulates (0–35%w/w) were produced by a manufacturing partner and shipped to a 3DP site in a different country.
Direct powder extrusion (DPE) 3DP was subsequently used to prepare printlets (3D printed tablets), with mass ranging 266–371 mg. All printlets released more than 80% drug load within the first 60 min of the in vitro drug release test. An in-line near-infrared spectroscopy system was used as a process analytical technology (PAT) to quantify the printlets’ drug load. Calibration models were developed using partial least squares regression, which showed excellent linearity (R2 = 0.9833) and accuracy (RMSE = 1.0662).
Overall, this work is the first to report the use of an in-line NIR system to perform real-time analysis of printlets prepared using pharma-inks produced by a pharmaceutical company. By demonstrating the feasibility of the proposed distribution model through this proof-of-concept study, this work paves the way for investigation of further PAT tools for quality control in 3DP point-of-care manufacturing.
Download the research paper as PDF: A case study on distributed manufacturing of 3D printed medicines
or read more here
Materials
All granulated formulations were supplied by Losan Pharma GmbH (Germany). The drug efavirenz was also provided by Losan for quality control and analytical purposes, and was obtained from Zhejiang Jiangbei Pharmaceutical Co., Ltd. (Zhejiang, China). Kollidon VA64 was donated by BASF (Ludwigshafen, Germany). Mannitol 100 was purchased from Merck KGaA (Darmstadt, Germany). Magnesium Stearate was purchased from Peter Greven Nederland C.V. (Venlo, The Netherlands).
Iria Seoane-Viaño, Xiaoyan Xu, Jun Jie Ong, Ahmed Teyeb, Simon Gaisford, André Campos-Álvarez, Anja Stulz, Carmen Marcuta, Lilia Kraschew, Wolfgang Mohr, Abdul W. Basit, Alvaro Goyanes, A case study on distributed manufacturing of 3D printed medicines, International Journal of Pharmaceutics: X, 2023, 100184, ISSN 2590-1567,
https://doi.org/10.1016/j.ijpx.2023.100184.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


