Application of Liquisolid Pellets Technology for Improving Dissolution of Posaconazole: A DoE Based Process Optimization
Abstract
Purpose
Posaconazole (PSZ) is BCS class-II drug that displays variable bioavailability upon oral administration due to extremely low and pH-dependent solubility.
Method
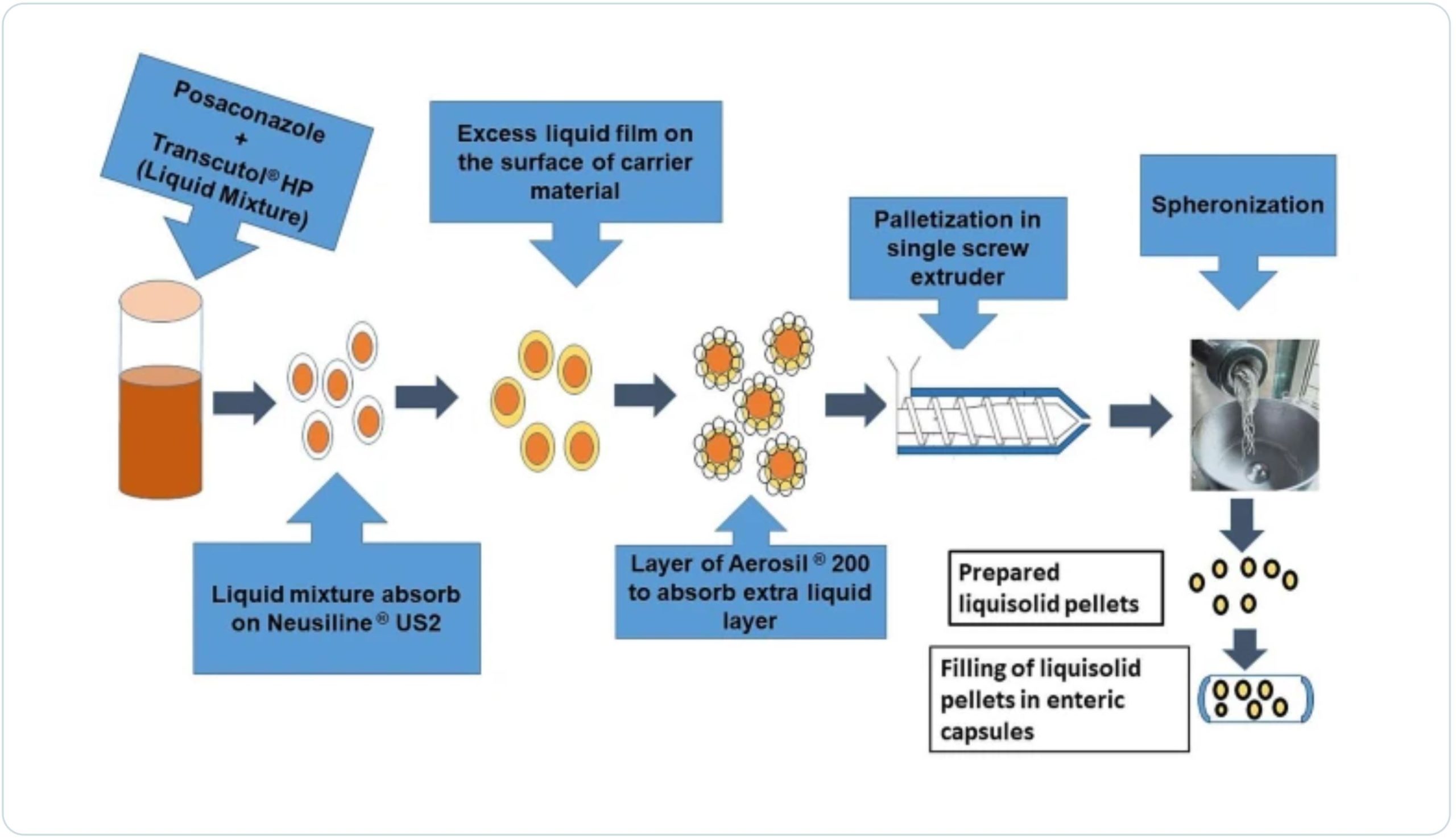
The present investigation was aimed to formulate and evaluate liquisolid pellets of PSZ for improving its dissolution. Liquisolid pellets were prepared using Transcutol® HP, Neusilin® US2, and Aerosil® 200 as non-volatile liquid, carrier, and coating materials respectively. A 32 full factorial design having excipient ratio (R) and spheronization speed as independent variables and the cumulative amount of drug dissolved at 135 min and 165 min as dependent variables were used for the process optimization.
Result
The results of regression analysis indicated a significant effect of selected independent variables on the dependent variables (p-value < 0.05). Differential scanning calorimetry (DSC) studies revealed that the PSZ remained in an amorphous or molecular dispersed state within the liquisolid pellets. Powder X-ray diffraction (PXRD) analysis indicated a significant reduction in crystallinity of the entrapped drug compared to pure drugs. The Fourier-transform infrared (FTIR) analysis demonstrated the stability of the drug within the optimized pellets. SEM images confirmed uniform and well-shaped spherical liquisolid pellets.
Conclusion
During In-vitro dissolution studies, the cumulative amount of the drug dissolved from the prepared pellets was less than 5% during the acid stage (750 ml 0.01 N HCL) whereas the improvement was significant during the buffer stage (750 ml 0.01 N HCL + 250 ml 0.2 M pH 6.8 Phosphate buffer + 1.46% polysorbate 80).
Read more here
Shah, S., Devani, P., Dudhat, K. et al. Application of Liquisolid Pellets Technology for Improving Dissolution of Posaconazole: A DoE Based Process Optimization. J Pharm Innov 19, 23 (2024). https://doi.org/10.1007/s12247-024-09830-0
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


