Effects of excipients on the interactions of self-emulsifying drug delivery systems with human blood plasma and plasma membranes
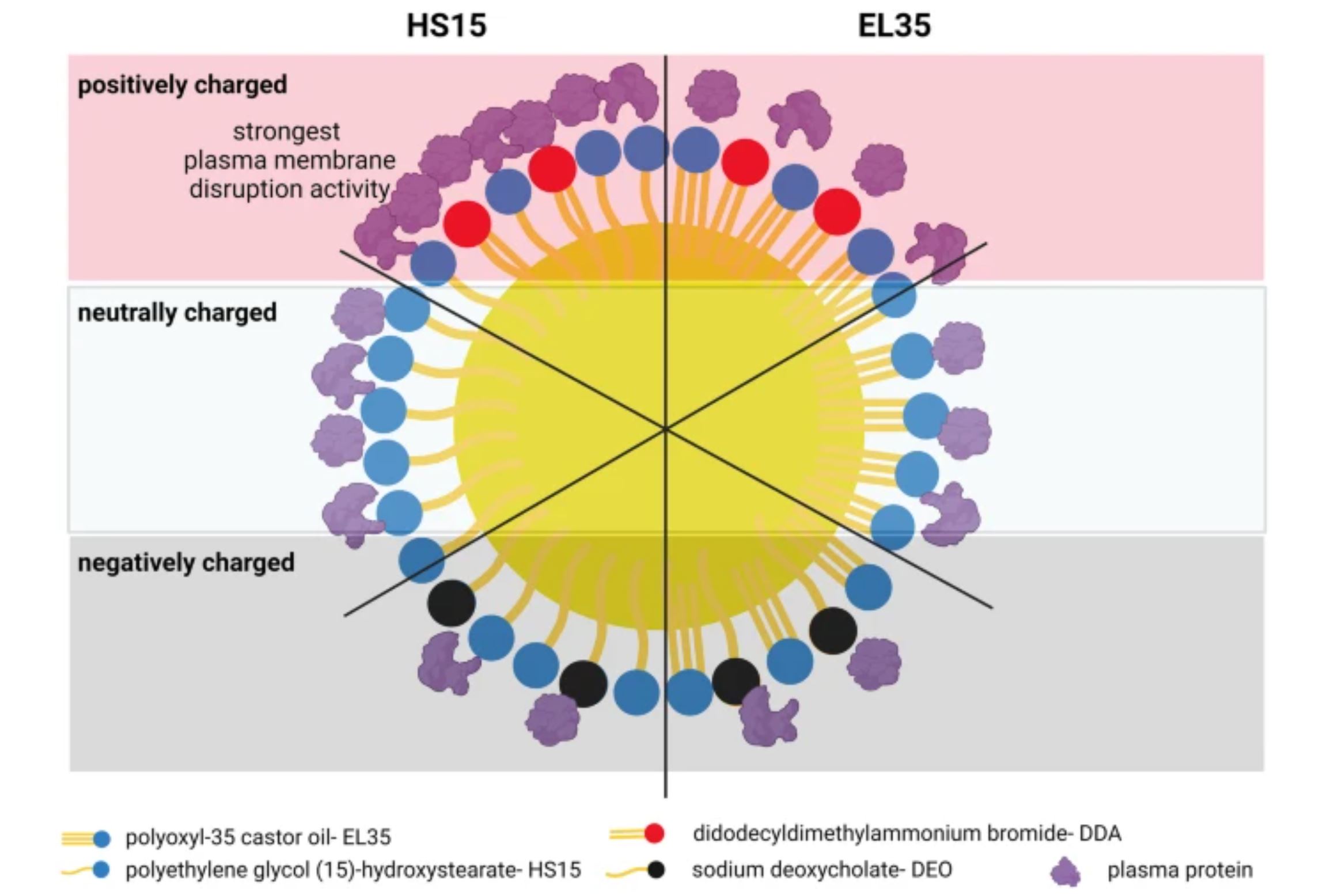
Due to its versatility in formulation and manufacturing, self-emulsifying drug delivery systems (SEDDS) can be used to design parenteral formulations. Therefore, it is necessary to understand the effects of excipients on the behavior of SEDDS formulations upon parenteral administration, particularly their interactions with blood plasma and cell membranes. In this study, we prepared three neutrally charged SEDDS formulations composed of medium-chain triglycerides as the oil phase, polyoxyl-35 castor oil (EL35) and polyethylene glycol (15)-hydroxystearate (HS15) as the nonionic surfactants, medium-chain mono- and diglycerides as the co-surfactant, and propylene glycol as the co-solvent.
The cationic surfactant, didodecyldimethylammonium bromide (DDA), and the anionic surfactant, sodium deoxycholate (DEO), were added to the neutral SEDDS preconcentrates to obtain cationic and anionic SEDDS, respectively. SEDDS were incubated with human blood plasma and recovered by size exclusion chromatography. Data showed that SEDDS emulsion droplets can bind plasma protein to different extents depending on their surface charge and surfactant used. At pH 7.4, the least protein binding was observed with anionic SEDDS. Positive charges increased protein binding. SEDDS stabilized by HS15 can adsorb more plasma protein and induce more plasma membrane disruption activity than SEDDS stabilized by EL35.
These effects were more pronounced with the HS15 + DDA combination. The addition of DDA and DEO to SEDDS increased plasma membrane disruption (PMD) activities, and DDA (1% w/w) was more active than DEO (2% w/w). PMD activities of SEDDS were concentration-dependent and vanished at appropriate dilution ratios.
Download the full article as PDF here: Effects of excipients on the interactions of self-emulsifying drug delivery systems with human blood plasma and plasma membranes
or read it here
Materials
Labrafac® Lipophile WL 1349 (MCT), mixture of medium-chain triglycerides of caprylic and capric acids, was kindly provided by Gattefossé. Capmul® MCM EP/NF (MCM), mixture of mono- and di-glycerides of caprylic and capric acids, was kindly provided by Abitec. Kolliphor® HS 15 (polyethylene glycol (15)-hydroxystearate- HS15), Kolliphor® EL (polyoxyl-35 castor oil- EL35), propylene glycol (PG), didodecyldimethylammonium bromide (DDA), sodium deoxycholate (DEO), sterile Dulbecco’s phosphate buffered saline pH 7.4 (PBS), fluorescein isothiocyanate isomer I (FITC) and pyrene were purchased from Sigma Aldrich, Austria. Sephacryl® S400-HR suspension (in 20% aqueous ethanol) with particle size 25–75 μm in wet state and fractionation range of globular proteins 2 × 104 – 8 × 106 was purchased from Sigma Aldrich, Austria. Micro BCA™ protein assay kit was purchased from Thermo Fisher Scientific, Austria. Human whole blood and frozen human plasma were kindly donated by the Blutbank, Tirol Kliniken GmbH, Innsbruck, Tirol, Austria. During whole blood donation, the anticoagulant solution CPD (citrate-phosphate-dextrose) was used to prevent clotting and maintain the shelf life of the blood products.
Le-Vinh, B., Le, NM.N., Phan, T.N.Q. et al. Effects of excipients on the interactions of self-emulsifying drug delivery systems with human blood plasma and plasma membranes. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01541-w
Read also our introduction article on Parenteral Excipients here:


