Exploring Environmental Settings to Improve the Printability of Paroxetine-Loaded Filaments by Fused Deposition Modelling
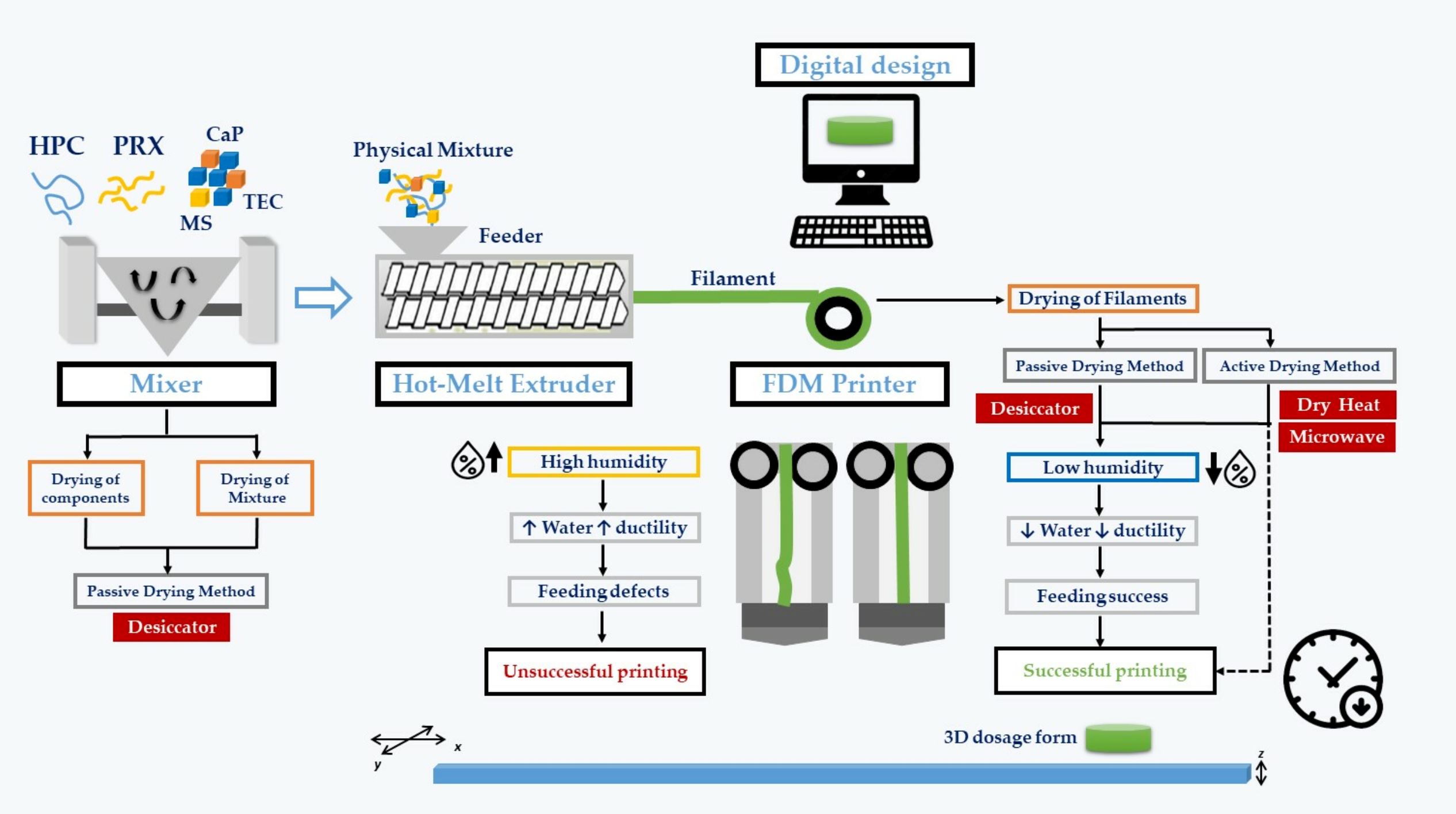
The successful integration of hot-melt extrusion (HME) and fused deposition modelling (FDM) depends on a better understanding of the impact of environmental conditions on the printability of formulations, since they significantly affect the properties of the raw materials, whose control is crucial to enable three-dimensional printing (3DP). Hence, the objective of this work was to investigate the correlation between the environmental settings and the properties of paroxetine (PRX)-loaded filaments, previously produced by HME, which affect printability by FDM.
The influence of different drying methods of the physical mixtures (PMs) and HME-filaments (FILs) on the quality and printability of these products was also assessed. The printability of FILs was evaluated in terms of the water content, and the mechanical and thermal properties of the products. Stability studies and physicochemical, thermal, and in vitro dissolution tests were carried out on the 3D-printed tablets. Stability studies demonstrated the high ductility of the PRX loaded FILs, especially under high humidity conditions. Under low humidity storage conditions (11% RH), the FILs became stiffer and were successfully used to feed the FDM printer.
Water removal was slow when carried out passively in a controlled atmosphere (desiccator) or accelerated by using active drying methods (heat or microwave). Pre-drying of the PRX/excipients and/or PMs did not show any positive effect on the printability of the FIL. On the contrary, dry heat and, preferably, microwave mediated drying processes were shown to reduce the holding time required for successful FDM printing, enabling on-demand production at the point of care.
Download the full article as PDF here Exploring Environmental Settings to Improve the Printability of Paroxetine-Loaded Filaments by Fused Deposition Modelling
or read it here
Materials
Paroxetine hydrochloride (Eur. Ph., melting point of 121–131 °C, Form II) was supplied by Lusifar (Lisbon, Portugal) and used as a model drug. Hydroxypropylcellulose (HPCTM LF Pharm, donated by Ashland Inc., Schaffhausen, Switzerland), triethylcitrate (TEC, Sigma Aldrich, Darmstadt, Germany), dicalcium dihydrate phosphate (CaP; Budenheim, Rheinstrasse, Germany) and magnesium stearate (MS; Roic Pharma, Terrasa, Barcelona, Spain) were used as excipients. Karl-Fisher titration was performed with Hydranal™- Composite 5 (Honeywell, Charlotte, NC, USA) and the different RH conditions were obtained with lithium chloride (Merck KGaA, Madrid, Spain). All other reagents were of analytical grade.
Figueiredo, S.; Fernandes, A.I.; Carvalho, F.G.; Pinto, J.F. Exploring Environmental Settings to Improve the Printability of Paroxetine-Loaded Filaments by Fused Deposition Modelling. Pharmaceutics 2023, 15, 2636. https://doi.org/10.3390/pharmaceutics15112636
Read also more on Magnesium Stearate as a pharmaceutical excipient here:


