Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing
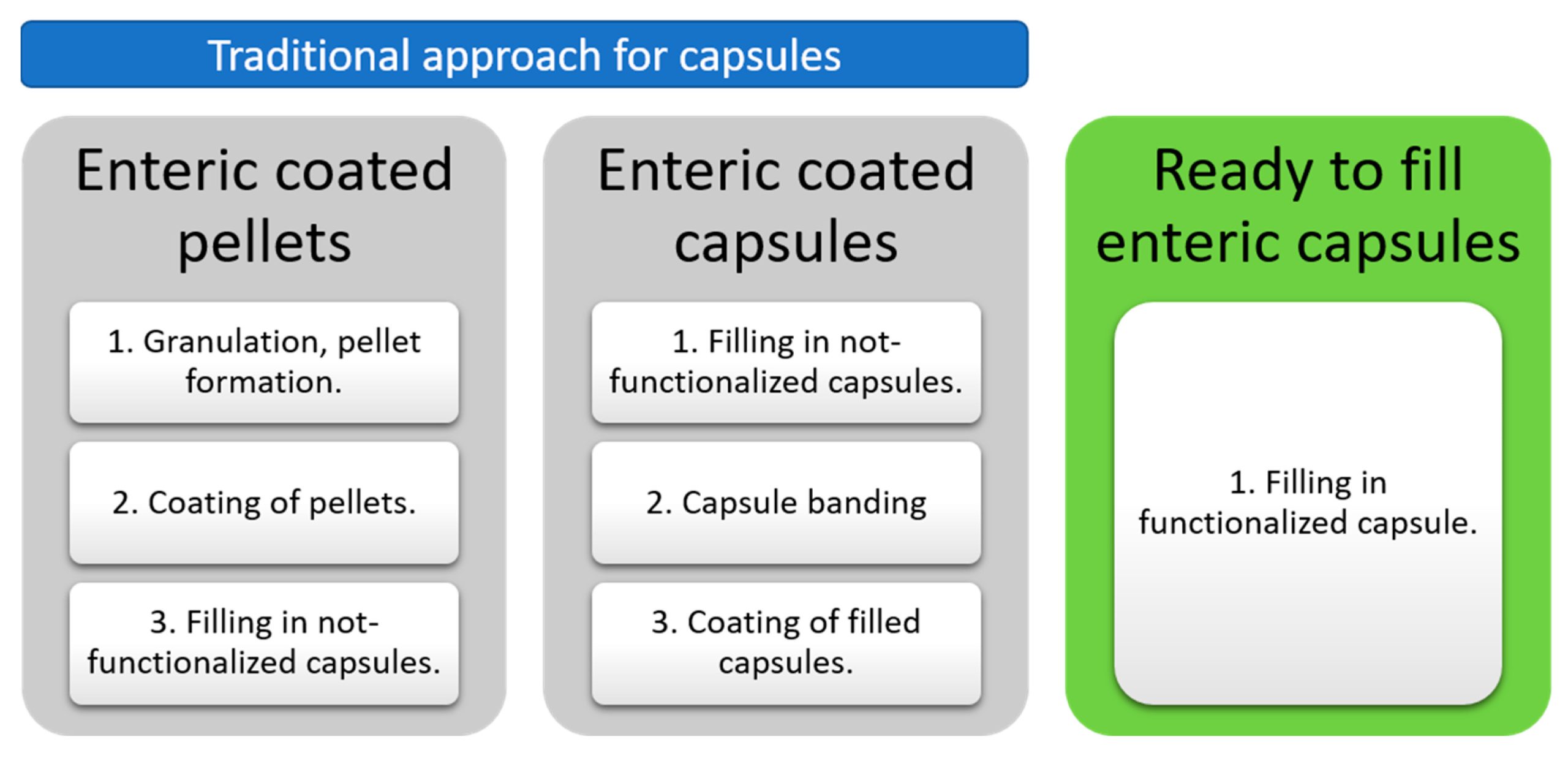
Developing delayed-release formulations for acid-sensitive actives can be a costly and time-consuming process. However, ready-to-fill functional capsules, such as EUDRACAP® can significantly mitigate these challenges. The in vitro performance of EUDRACAP® enteric was evaluated in two typical delayed-release scenarios: for diclofenac (a drug that can cause irritation to gastric mucosa), and for omeprazole (a drug susceptible to degradation due to the acidity of gastric fluid). The prototypes were tested in HCl 0.1N according to the USP <711> for at least 2 h and compared to commercial products. The results showed that the performance of EUDRACAP® was below LOD and in compliance with the requirements for drug release in acidic media (NMT 10%). Additionally, the impurities were evaluated after the acidic stress. The low total percentage of impurities of 0.44% for diclofenac (NMT 1.50%) and 0.22% for omeprazole (NMT 2.00%) indicates a very good protection by EUDRACAP®. A comprehensive comparative analysis of the in vitro performance clearly showed the acid protection capability of EUDRACAP® enteric capsules making them a serious alternative to existing enteric dosage forms alternatives. EUDRACAP® is an accessible solution both in large-scale industrial and smaller pharmacy settings. Offering increased accessibility, affordability, and convenience to manufacturers and consumers alike and leading to improved healthcare outcomes.
1. Introduction
2.2. Standards, Samples and Excipients
or read it here
Afonso Urich, J.A.; Fedorko, A.; Hölzer, B.; Khinast, J. Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing. Pharmaceutics 2023, 15, 2592.
https://doi.org/10.3390/pharmaceutics15112592

