Study of Powder and Tableting Functionality Towards Evaluation and Characterisation of BARETab® PH (all in one excipient) as a Substitute of Conventional Physically Mixed Microcrystalline Cellulose, Croscarmellose Sodium, Silicon Dioxide and Purified Talc in a PCM Formulation
Abstract
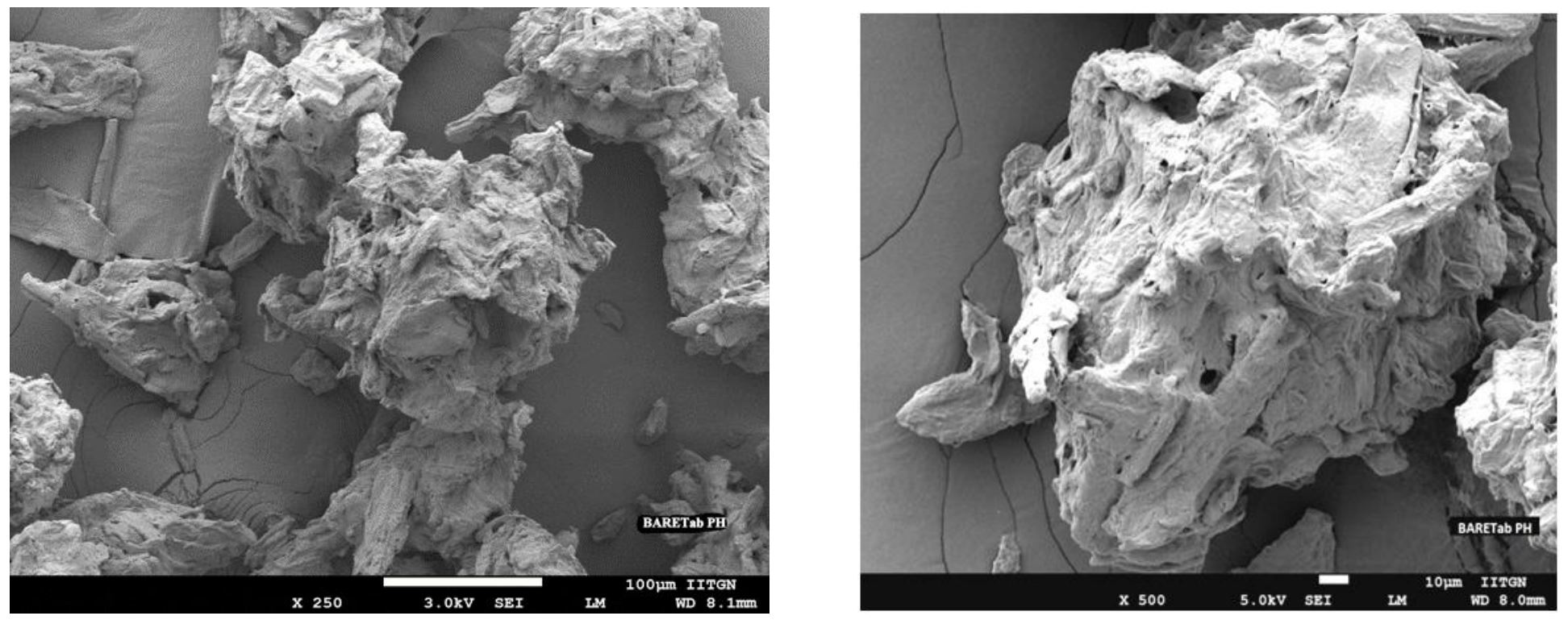
BARETab® PH is a multi-functional ready to use premixed, co-processed ingredient for direct compression (DC) formulations. It is a combination of selective, largely used excipients for DC. Direct compression is the most widely used tableting method because of the simple manufacturing process with higher output in a shorter time. In DC formulations, the major disadvantage is due to heterogeneous mixture of excipients and API. Mostly APIs have poor flowability and compressibility, which cause weight, hardness variation, high friability and unequal API distribution in the tablets. In general, excipients and APIs have different particle size which makes heterogeneous mixture challenging.
Fine particles of API and excipient cause physical defects like sticking, capping and lamination in formulations. BARETab® PH can solve all these problems primarily due to its homogeneous mixing, larger surface area and superior flowability which carries the API and allows equal distribution in the tablets. BARETab® PH facilitates better uniformity, higher tablet hardness, shorter disintegration time and eliminates physical tableting defects. In this study, we have produced Paracetamol (PCM) tablet by direct compression with improved tablet properties and shortened manufacturing time when compared to the wet granulation method.
Download the full article as PDF here STUDY OF POWDER AND TABLETING FUNCTIONALITY TOWARDS EVALUATION AND CHARACTERISATION OF BARETab® PH (ALL IN ONE EXCIPIENT) AS A SUBSTITUTE OF CONVENTIONAL PHYSICALLY MIXED MICROCRYSTALLINE CELLULOSE, CROSCARMELLOSE SODIUM, SILICON DIOXIDE AND PURIFIED TALC IN A PCM FORMULATION
Material
BARETab® PH, HiCelTM filler, binder and HiLoseTM disintegrant manufactured at Sigachi Industries Ltd. in Dahej, Gujarat. Glidant purchased form Nikon Corporation, Wacker Germany, lubricant purchased from Gangotri Inorganics (P) Ltd and Paracetamol purchased form Farmson pharmaceuticals, Gujarat.
Source: Sigachi, Journal Of Harmonized Research in Pharmacy, 9(2), 2020, 27-36, STUDY OF POWDER AND TABLETING FUNCTIONALITY TOWARDS EVALUATION AND CHARACTERISATION OF BARETab® PH (ALL IN ONE EXCIPIENT) AS A SUBSTITUTE OF CONVENTIONAL PHYSICALLY MIXED MICROCRYSTALLINE CELLULOSE, CROSCARMELLOSE SODIUM, SILICON DIOXIDE AND PURIFIED TALC IN A PCM FORMULATION, Monika Tomar, Amit Raj Sinha, Sigachi® Industries Limited, Dahej SEZ, Bharuch, Gujarat, India. Sigachi Industries Limited, Kalayan’s Tulsiram Chambers, Madinaguda, Hyderabad, Telangana (India), Received on: March 2020, Accepted after revision: April 2020
Do you need more information or a sample of Sigachi excipients?

