The effect of excipient particle size on the reduction of compactibility after roller compaction
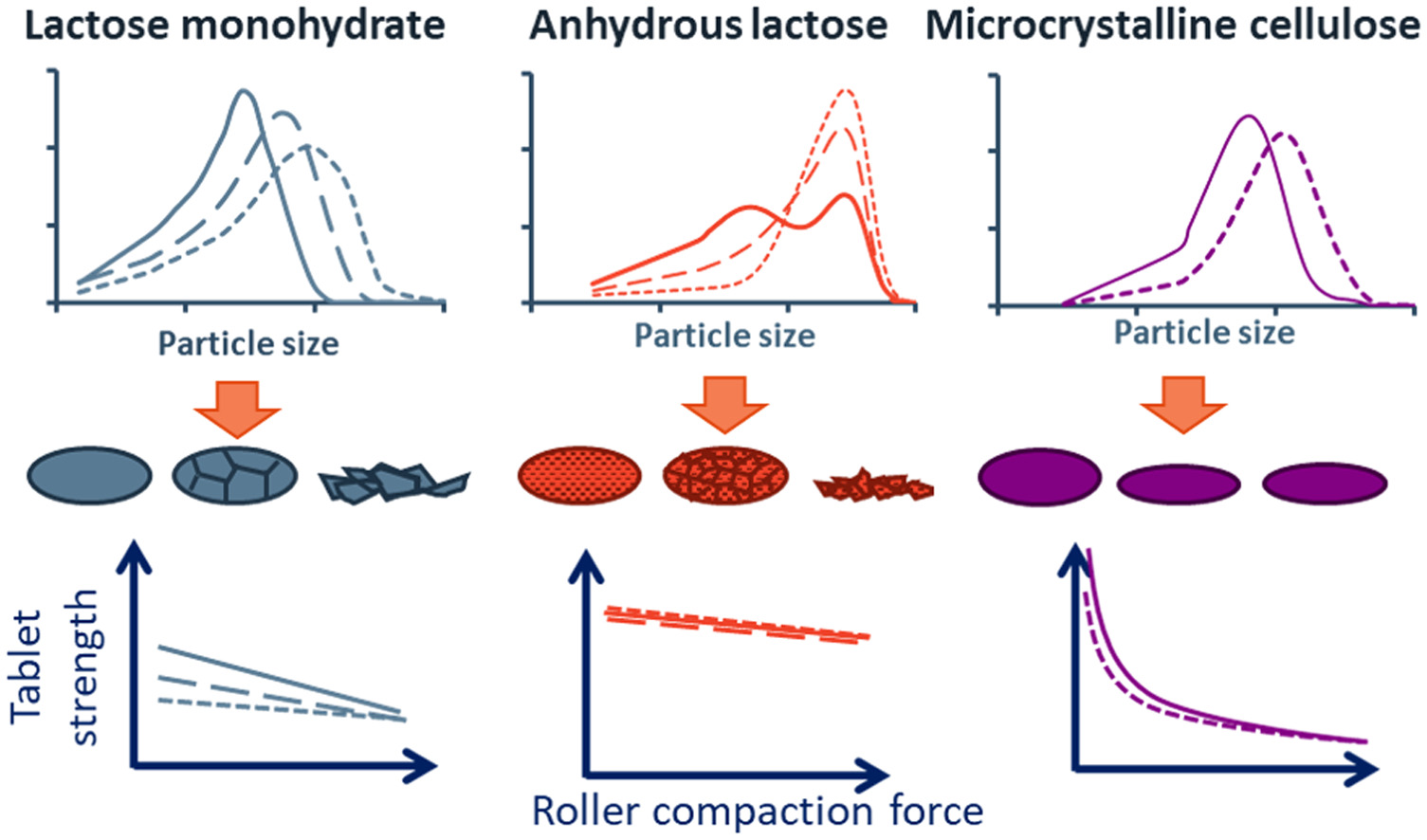
Developing a robust roller compaction process can be challenging, due to the diversity in process parameters and material properties of the components in a formulation. A major challenge in dry granulation is the reduction of tablet strength as a result of re-compaction of the materials. The aim of this study is to investigate the impact of excipient type and particle size distribution on tablet tensile strength after roller compaction.
Highlights
- • The impact of particle size on the re-compactibility differs per excipient type.
- • Re-compactibility is classified and quantified for different excipients.
- • Rough surfaces and a high degree of fragmentation are beneficial for re-compaction.
- • The particle size of anhydrous lactose hardly affects the re-compactibility.
Lactose monohydrate, anhydrous lactose and microcrystalline cellulose with different particle sizes are roller compacted at varying specific compaction forces. Granules obtained are compressed into tablets to evaluate the reduction in tablet strength upon increasing the specific compaction force. The impact of particle size of the starting material is shown to be vastly different for the three types of excipients investigated, due to the differences in mechanical deformation mechanisms. The presence of rough surfaces and a high degree of fragmentation for anhydrous lactose appears to be beneficial for compaction and re-compaction process. Additionally, the particle size of anhydrous lactose hardly affects the tensile strength of tablets, which can be beneficial for the robustness of a roller compaction process.
Continue reading here
About this article: Pauline H.M. Janssen, Maarten Jaspers, Robin Meier, Timo P. Roelofs, Bastiaan H.J. Dickhoff,
The effect of excipient particle size on the reduction of compactibility after roller compaction, International Journal of Pharmaceutics: X, Volume 4, 2022, 100117, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2022.100117.
(https://www.sciencedirect.com/science/article/pii/S2590156722000068)
Materials
Anhydrous lactose (SuperTab® 21AN, SuperTab® 22AN, Lactopress® anhydrous fines), milled lactose monohydrate (Pharmatose® 150 M, Pharmatose® 200 M, Pharmatose® 350 M) and microcrystalline cellulose (Pharmacel® 101, Pharmacel® 102) were obtained from DFE Pharma (Goch, Germany). Before roller compaction, materials were dry blended with 4% w/w croscarmellose sodium (Primellose®, DFE Pharma, Goch, Germany) in a PM 600 (L.B. Bohle, Ennigerloh, Germany) bin blender at 6 rpm for 15 min.

