Development of multiple structured extended release tablets via hot melt extrusion and dual-nozzle fused deposition modeling 3D printing
Abstract
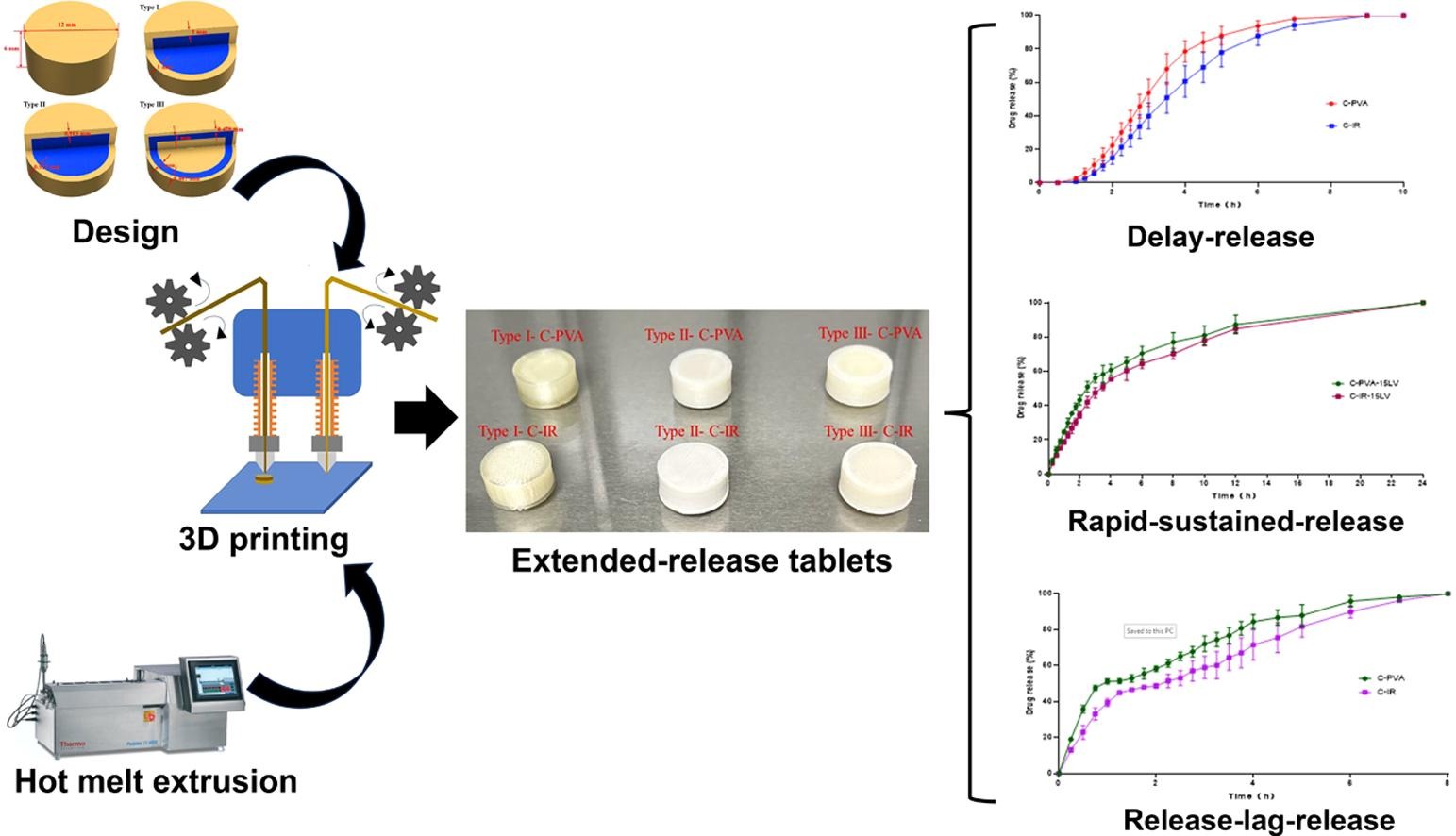
The study aims to fabricate extended release (ER) tablets using a dual-nozzle fused deposition modeling (FDM) three-dimensional (3D) printing technology based on hot melt extrusion (HME), using caffeine as the model compound. Three different ER tablets were developed, which obtained “delayed-release”, “rapid-sustained release”, and “release-lag-release” properties. Each type of tablet was printed with two different formulations. A novel printing method was employed in this study, which is to push the HME filament from behind with polylactic acid (PLA) to prevent sample damage by gears during the printing process. Powder X-ray diffractometry (PXRD) and differential scanning calorimetry (DSC) results showed that caffeine was predominately amorphous in the final tablets. The dissolution of 3D printed tablets was assessed using a USP-II dissolution apparatus. ER tablets containing PVA dissolved faster than those developed with Kollicoat IR. Overall, this study revealed that ER tablets were successfully manufactured through HME paired with dual-nozzle FDM 3D printing and demonstrated the power of 3D printing in developing multi-layer tablets with complex structures.
Highlights
- Fused Deposition Modeling 3D printing was used to prepare extended release tablets with different release profiles.
- A novel printing method for Bowden printer was developed.
- The combination of hot melt extrusion and 3D printing is a promising approach to formulating multi-layer tablets with complex structures.
Introduction
As an emerging manufacturing technology for pharmaceutical dosage forms, three-dimensional (3D) printing stands out. 3D printing, a type of additive manufacturing (AM) technique, creates objects with intricate structures by depositing materials layer by layer from a digital design, guided by computer-aided design (CAD) (Pucci et al., 2017, Wang et al., 2023). The advantages of manufacturing tablets using 3D printing technology encompass the ability to customize dosage forms to individual patient needs, precise control over the spatial distribution of the active pharmaceutical ingredient (API) in the dosage form, production of complex geometries, waste reduction, and swift fabrication of varying compositions for screening campaigns or individualized dose strength (Dumpa et al., 2021, Chakka and Chede, 2023, Prasad and Smyth, 2016). Therefore, 3D printing reveals immense potential for crafting personalized drug delivery systems, attributed to its exceptional flexibility and fabrication capabilities (Feng and Repka, 2024, Zhang et al., 2023).
Fused-deposition modeling (FDM) remains the most prevalent 3D printing technology, favored for its affordability and user-friendliness (Nyavanandi et al., 2022, Fina et al., 2017). FDM 3D printing is an extrusion-based technique wherein filaments are heated to a semi-solid state, then extruded and solidified onto a printing platform in layers (Chung et al., 2022, Goyanes et al., 2015). There are two main types of FDM 3D printing methods: direct and Bowden extrusion FDM (Chung et al., 2023). The primary distinction between these methods lies in the setup of the motor drive gear and the heater. In direct extrusion printers, the motor drive gear is right above the heater. Conversely, in Bowden extrusion printers, this gear is distant from the heater, drawing the filament into the heater via a long PTFE Bowden tube (Tlegenov et al., 2018, Zhang et al., 2022). Bowden extrusion printers, due to their lightweight print head and reduced inertia during movement, offer higher precision than their direct extrusion printer (Xu et al., 2020). This study utilized the dual-nozzle Bowden extrusion FDM method. Given that the filament feeding process in FDM is openly accessible, hot-melt extrusion (HME) is often employed in the pharmaceutical domain to produce filaments comprising both API and polymer (Nasereddin et al., 2018, Prasad and Smyth, 2016, Okafor-Muo et al., 2020).
Over the past two decades, HME has been widely used as a technique to manufacture various solid oral dosage forms (Narala et al., 2023, Dos Santos et al., 2021). HME is a process in which API-polymer blend is extruded through a die into a product of uniform shape at extrusion temperature and shear force (Patil et al., 2024, Wilson et al., 2012). During the extrusion process, the API is dispersed in the polymer to form a solid dispersion (Althobaiti et al., 2023, Alzahrani et al., 2022b, Tiwari et al., 2016). HME is a continuous, solvent-free process with the ease of scalability (Almutairi et al., 2022, Zhang et al., 2020, Censi et al., 2018). HME has been widely used in the production of different drug delivery systems such as tablets, implants and transdermal formulations (Raman Kallakunta et al., 2023, Alzahrani et al., 2023). Recently, the combination of HME and FDM 3D printing technology has gained significant attention, giving greater impetus to the application of HME in pharmaceuticals (Dos Santos et al., 2021, Almotairy et al., 2023, Nyavanandi et al., 2024).
Extended release (ER) formulations are a type of oral dosage form designed to release the API over a prolonged time period in the gastrointestinal tract. This gradual release mechanism is achieved through various formulation strategies, which allow the medication to be slowly absorbed (DiFranco Nicholas, 2023). There are many advantages of oral ER formulations, such as (1) improved patient compliance, (2) reduced side effects, and (3) an enhanced therapeutic effect (Andrade, 2015). However, the preparation of ER tablets through the conventional method is associated with some challenges, for example, the complexity of manufacturing steps, the requirement for a large amount of excipients, and the overly simplistic release profiles (Venkateswarlu et al., 2017). To overcome these issues, FDM 3D printing technology has been applied to provide a relatively convenient manufacturing process, allowing different drug release profiles during dissolution (Gültekin et al., 2021).
The present study aims to demonstrate the flexibility of HME and FDM 3D printing technology in the development of ER tablets. Due to the presence of two different components in each tablet, a dual-nozzle FDM 3D printer was employed. Three different types of ER tablets were formulated, which obtained “delayed-release” (Type I), “rapid-sustained release” (Type II), and “release-lag-release” (Type III) release profiles. Two different formulations were developed and compared for each type of tablet. The dissolution performance of the ER tablets was also evaluated.
Read more here
Materials
Caffeine was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Affinisol™ HPMC HME 15LV (molecular weight = 85 kDa) was donated by Colorcon, Inc. (PA, USA). Parteck® MXP polyvinyl alcohol (PVA) EMPROVE® ESSENTIAL (molecular weight = 32 kDa) and Parteck® SI 150 (sorbitol) were graciously supplied by EMD Millipore (Burlington, MA, USA). Kollicoat® IR (polyethylene glycol and polyvinyl alcohol copolymer; IR) and Kollidon® VA64 (vinylpyrrolidone-vinyl acetate copolymer; VA64) were provided by BASF
Peilun Zhang, Jinghan Li, Eman A. Ashour, Sooyeon Chung, Honghe Wang, Sateesh Kumar Vemula, Michael A. Repka, Development of multiple structured extended release tablets via hot melt extrusion and dual-nozzle fused deposition modeling 3D printing, International Journal of Pharmaceutics, Volume 653, 2024, 123905, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123905.
Read more on Sorbitol here:


