In situ triggered, floating delivery systems of capsaicin for prolonged gastroprotection
Abstract
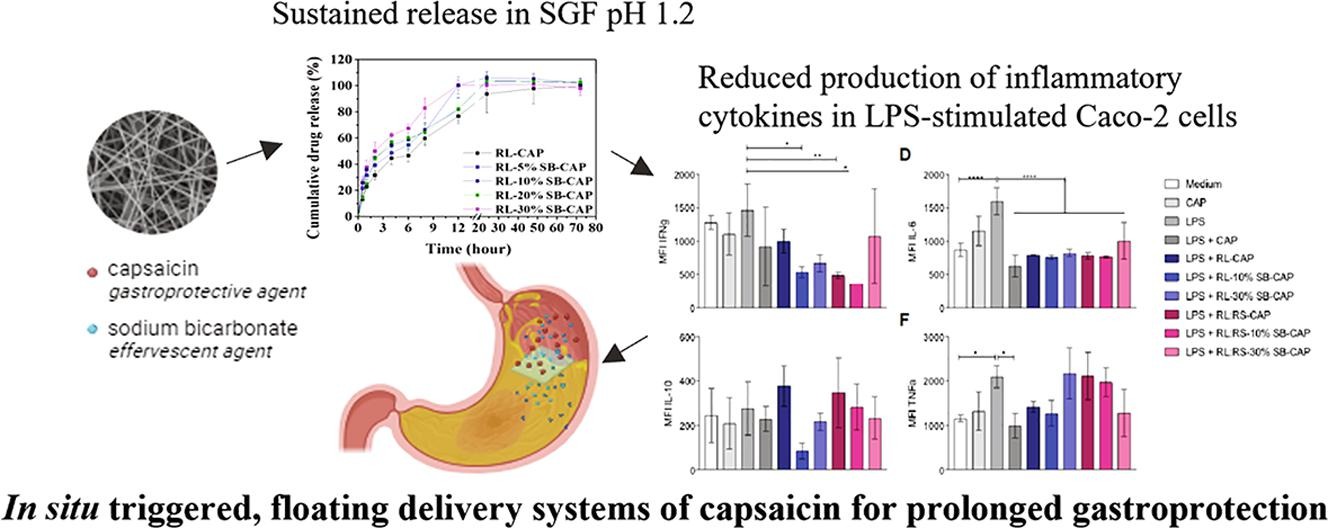
Capsaicin (CAP) has been implicated as a gastroprotective agent in the treatment of peptic ulcers. However, its oral administration is hampered by its poor aqueous solubility and caustic effect at high administered doses. To address these limitations, we describe the development of gastric floating, sustained release electrospun films loaded with CAP. The nanofiber films were formulated using the polymers Eudragit RL/RS and sodium bicarbonate (SB) as the effervescent agent. The films were tested for their physicochemical properties, and film buoyancy and in vitro release of CAP were assessed in simulated gastric fluid. The cytocompatibility and anti-inflammatory properties of the films were evaluated in lipopolysaccharide (LPS)-stimulated Caco-2 cells. The amorphous films showed improved wettability, a short floating lag time (<1 s) and a total floating time of over 24 h accompanied by sustained CAP release for up to 24 h. CAP-loaded films demonstrated biocompatibility with Caco-2 cells and potential cytoprotective effects by attenuating inflammatory cytokine and reactive oxygen species (ROS) production in LPS-stimulated Caco-2 cells. The gastric floating electrospun films could serve as a platform for sustained and stomach-specific drug delivery applications.
Introduction
Peptic ulcer disease (PUD) is one of the most common digestive disorder affecting the mucosal integrity of the stomach and proximal duodenum [1]. Its pathophysiology generally stems from an imbalance between the aggressive factors causing PUD and the protective mechanisms of the gastric mucosa and is associated with various changes in physiological parameters that may induce oxidative stress, lipid peroxidation and excessive gastric acid secretion. Among the most common causes of PUD are the chronic use of non-steroidal anti-inflammatory drugs (NSAIDs), Helicobacter pylori (H. pylori) infection and increased activity of the gastric secretory cells (Zolliger-Ellison syndrome) [2]. Owing to the fact that approximately 70 % of PUD cases may be asymptomatic or exert nonspecific symptoms, diagnosis and treatment require clinical attention to avoid PUD-related complications including hemorrhage, perforation and gastric obstruction [3].
Eradication of H. pylori infection is the standard of care for the management of PUD. However, the global prevalence of H. pylori antibiotic resistance increases the risk of gastric cancer and the emergence of new multidrug-resistant bacterial strains [4]. With the risk factors of PUD experiencing a gradual shift from bacterial-related to drug-related cases, the use of H2-antagonists and proton pump inhibitors (PPIs) has increased [5]. Although antisecretory drugs have been used for years to treat gastric disorders, long-term PPI treatment may be associated with certain medication risks, such as elevated risk of fracture [6]. In search of alternative gastroprotective agents of natural origin, curcumin [7] and CAP [2] have been identified for their anti-ulcer effects through the attenuation of different ulcerative effectors, which are further complimented by fewer side effects and a significantly lower cost.
An ideal gastroprotective strategy should enable prolonged retention and sustained drug release within the gastric environment. Floating delivery systems (FDS) are designed to remain in the stomach for an extended period, which could be advantageous for drugs that need to act locally in the gastric region allowing for sustained drug release and increased drug exposure to the stomach lining [8], [9]. FDS achieve buoyancy through polymers that upon ingestion swell in the stomach and trap the produced gases [8] or gas-generating agents, such as sodium bicarbonate (SB, NaHCO3), tartaric acid and citric acid [10].
CAP is a Biopharmaceutics Classification System (BCS) class II compound with poor aqueous solubility and low dissolution rate. Oral formulation approaches have been mainly focused on improving its aqueous solubility and bioavailability through the development of polymeric nanoparticles based on methoxy poly (ethylene glycol)-poly(ε-caprolactone) [11] and lipid multi-particulate formulations [12]. CAP-loaded electrospun films based on poly-L-lactic acid and gelatin have been also developed for the topical treatment of mouth ulcers [13]. Due to its caustic effect, sustained release of low CAP doses is highly desirable, especially in the treatment of gastric ulcers. To date, there has been no study that has comprehensively addressed the challenges associated with the limited solubility of CAP, the requirement for sustained release, and the prolongation of gastric residence when it comes to treating peptic ulcers. In this study, we developed in situ triggered, effervescent, floating electrospun nanofiber films for the oral delivery and sustained release of CAP in the gastric environment for the treatment of peptic ulcers (Fig. 1). FDS were developed based on polymers that can provide sustained drug release (Eudragit RL PO and Eudragit RS 100), using SB as effervescent agent and CAP as the gastroprotective agent. Eudragit RL and RS are water insoluble polymers with pH independent swelling. Eudragit RL has a much higher water permeability compared to Eudragit RS, due to the presence of a much higher content of quaternary ammonium groups in its structure [14], [15]. They are widely used in pharmaceutical manufacturing, often in combination and at different ratios, for achieving customized sustained drug release profiles across the gastrointestinal tract, that can help decrease the frequency of drug administration and therefore improve patient compliance [14], [16], [17], [18]. The floating profile of the films and release of CAP from the films were monitored in simulated gastric fluid. The compatibility and potential gastroprotective action of the CAP-loaded floating films were assessed in Caco-2 cells after LPS stimulation by monitoring changes in the levels of cytokine and reactive oxygen species (ROS) production.
Read more here
Materials
Sodium bicarbonate (≥99.7 %, NaHCO3, SB), acetone (≥99.5 %), N,N-Dimethylformamide (anhydrous, 99.8 %, DMF), Tween 80, hydrochloric acid (37 %) and Capsaicin (CAP ≥ 98.5 %, analytical standard) were purchased from Sigma-Aldrich (Darmstadt, Germany). Eudragit RL PO (RL) and Eudragit RS 100 (RS) were provided by Evonik Industries AG (Essen, Germany).
Konstantina Chachlioutaki, Pedro H.D.M. Prazeres, Sérgio R.A. Scalzo, Pelagia Bakirtzi, Samson Afewerki, Pedro P.G. Guimaraes, Nikolaos Bouropoulos, Dimitrios G. Fatouros, Christina Karavasili, In situ triggered, floating delivery systems of capsaicin for prolonged gastroprotection, European Journal of Pharmaceutics and Biopharmaceutics, Volume 197, 2024, 114212, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2024.114212.
Read more on Orally Disintegrating Tablets (ODTs) here:


