A new stable and bioactive formulation of Geniotrigona thoracia propolis microemulsion for oral delivery
Abstract
Propolis, particularly those produced by stingless bees, is known to have a broad range of medicinal benefits due to its antioxidant properties. However, its strong and unpleasant flavour and sticky texture make it less palatable. This study aimed to improve the oral administration of stingless bee propolis extract for nutraceutical use by formulating it into a microemulsion. The microemulsion was created using a water titration method and a pseudo-ternary phase diagram to determine the optimal surfactant: co-surfactant ratio. The formulation was then characterized for its globule size, pH, viscosity, and phenolic content, and subjected to stability tests under ambient and accelerated conditions. Anti-microbial and antioxidant activity were evaluated through the disk diffusion method and the ABTS assay. The microemulsion had an average droplet size of 229.2 nm, a pH of 4.78 ± 0.18, and a viscosity of 0.149 Pa, which falls within the typical range for oral formulations. The microemulsion successfully preserved the antioxidant and antibacterial properties of the propolis extract. Long-term and accelerated stability studies indicated that the microemulsion’s pH, appearance, and viscosity remained stable for up to 12 months, although there was a 24 % loss in phenolic activity over the same period. Overall, a stable and potent propolis microemulsion was successfully developed using pharmaceutical and food-grade ingredients, providing a safe and effective way to orally deliver propolis.
Highlights
First study on Malaysian Kelulut propolis microemulsion formulation for oral consumption.
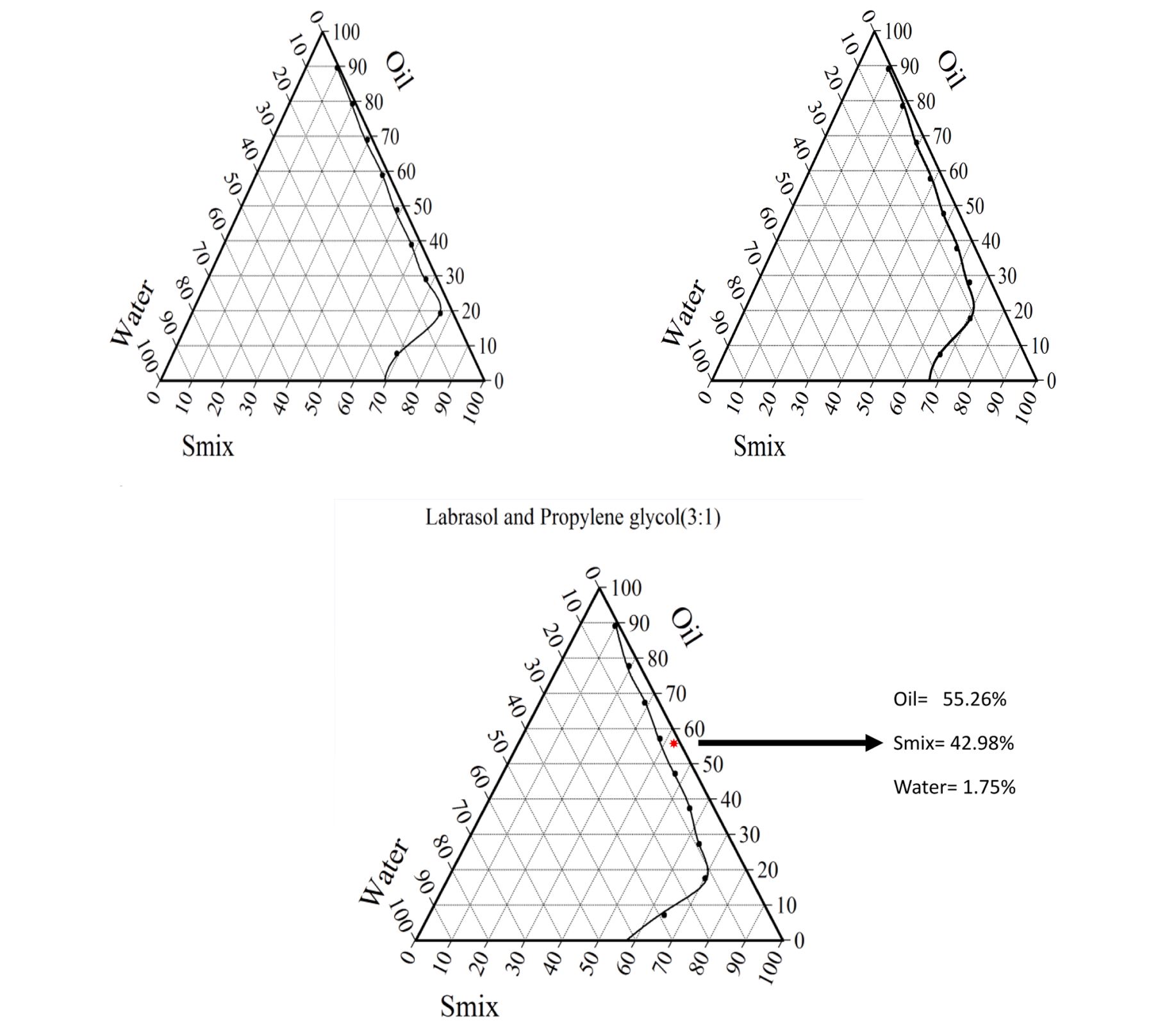
- Formulation utilized non-ionic surfactants (i.e., Labrasol) which have low toxicity.
- Other formulation ingredients are generally recognized as safe (oleic acid, propylene glycol).
- Phenolics retained for up to 6 months under both ambient and accelerated stability conditions.
- Formulated microemulsion possessed good antibacterial and antioxidant properties.
Introduction
Propolis is a resinous substance that bees collect from the leaf buds and bark of poplar trees or other species (Maroof & Gan, 2021). Folk medicines in many regions worldwide have used propolis for its biological and pharmacological properties, including hepatoprotective effects, antitumor, antioxidative, antimicrobial, and anti-inflammatory effects (Silva-Carvalho et al., 2015; Maroof et al., 2020). Due to these properties, propolis is commonly applied in food, beverages, cosmetics, and medicine for improving health and preventing diseases (Alam et al., 2023; Irigoiti et al., 2021).
Stingless bees are widespread and well-adapted to tropical and subtropical regions of the world, such as Central and South America, Africa, Northern Australia, and Southeast Asia (Maroof et al., 2023; Ivorra et al., 2020). In Malaysia, approximately 40 different indigenous species of stingless bees, locally known as “lebah kelulut”, exist, but stingless bee culture in Asia are mostly from two domesticated native species: Geniotrigona thoracica (G. thoracica) and Heterotrigona itama (H. itama) (Ivorra et al., 2020).
Although more than 800 constituents have been identified in propolis (Kasote et al., 2022), the functional properties are mainly attributed to the polyphenols of flavonoids, phenolic acids, and their esters (Maroof & Gan, 2022; Galeotti et al., 2018). However, raw propolis is difficult to use due to its low water solubility, susceptibility to oxidation, low bioavailability, and short half-life, limiting its use in humans unless suitably formulated (Fan et al., 2014). To be utilized for human consumption, raw propolis (which contains wax and other insoluble materials) must first undergo extraction to yield an extract containing the biologically active constituents. However, upon extraction, the bitter taste,low water solubility and low thermal and oxidative stability of the extracts raise some challenges for food applications and consumer acceptance (Maroof et al., 2023).
Microemulsions (MEs) have generated considerable interest over the years as a good drug delivery system (Pavoni et al., 2020). MEs are colloidal, optically isotropic, transparent or slightly opalescent formulations of low viscosity consisting of surfactant, co-surfactant, oil and water (Fan et al., 2014). They have advantages such as thermodynamic stability, ease of preparation, good solubilization capacity of lipophilic, hydrophilic, and amphiphilic solutes, making them very versatile (Tartaro et al., 2020). MEs have emerged as novel vehicles for drug delivery via multiple routes, including transdermal, topical, oral, and parenteral, allowing for more targeted, sustained, or controlled release formulations that can improve therapeutic outcomes and reduce drug toxicity (Pavoni et al., 2020). For natural products developed into nutraceuticals, MEs can aid in taste masking and enhance the antimicrobial activity of formulations (Seibert et al., 2019).
Emulsions loaded with propolis extract (PE) have been developed for several applications. Žilius et al. (2016) created a ME using PEG-8 caprylic/capric glycerides (labrasol) and ethanolic PEs, with the goal of delivering phenolic components present in propolis ex vivo into the skin. In this oil in water ME, the phenolic compounds were mostly present in the ME lipid phase, thus limiting their availability at the skin’s surface. Nevertheless, the formulation is viewed as a good source of antioxidant with reduced potential to cause oxidative stress on biological systems.
In another study, Fan et al. (2014) explored if the immune-enhancing properties of propolis flavonoids (PF’s) might be improved by converting it to a propolis ME. When compared to PF’s alone, their findings demonstrated that propolis ME (at medium and high doses of PFs) dramatically boosts immunological organ indices, overcomes immunosuppression, accelerates lymphocyte proliferation, and improves serum interleukin concentrations. Consequently, ME may be a useful formulation that can enhance PF’s bioavailability. Dantas et al. (2010), used ethanolic extracts of Brazilian propolis incorporated into MEs for topical application, and optimized the formulation by varying compositions of Tween 20, Tween 80, ethanol, and water. The MEs exhibited antimicrobial activity against gram positive bacteria.
However, studies on the development of oral propolis MEs are limited. Such formulations are important since the oral route is the most common delivery route for natural product supplements. We have already conducted and published a thorough evaluation of the propolis extract in terms of the individual compounds present, total phenolics and flavonoid content (Maroof et al., 2023). In this study, we aim to formulate a ME of the characterized stingless bees G. thoracica propolis for oral administration as a health supplement using food and pharmaceutical grade components. We present a systematic screening of ME components in the formulation to guide selection of the final formulation combinations. The step is followed by an extensive physical, chemical and biological characterization of the formulation as well as long term stability studies to guide its commercial potential.
Download the full article as PDF here A new stable and bioactive formulation of Geniotrigona thoracia propolis microemulsion for oral delivery
or read it here
Materials
Folin-Ciocalteu reagent, sodium carbonate, gallic acid were purchased from Sigma-Aldrich (Germany). Ethanol was obtained from Merck (Germany). Food grade glycerol, propylene glycol, Tween 20 and Tween 80 were purchased from Sigma-Aldrich (Germany). Oral pharmaceutical grade polyoxyl 35 castor oil (Kolliphor EL) and polyoxyl 40 hydrogenated castor oil (Kolliphor RH40) were purchased from BASF (Ludwigshafen, Germany). Pharmaceutical grade Labrasol was purchased from Gattefossé (Saint-Priest, France).
Kashif Maroof, Ronald F.S. Lee, Lee Fong Siow, Bey Hing Goh, Ken Fong Chen, Siew Hua Gan, A new stable and bioactive formulation of Geniotrigona thoracia propolis microemulsion for oral delivery, Food Chemistry Advances,
Volume 3, 2023, 100514, ISSN 2772-753X, https://doi.org/10.1016/j.focha.2023.100514.
Read also our article Orally Disintegrating Tablets (ODTs) here:


