Impact of two-step glidant mixing process on flow performance of coated curcumin – in vitro, in vivo investigation
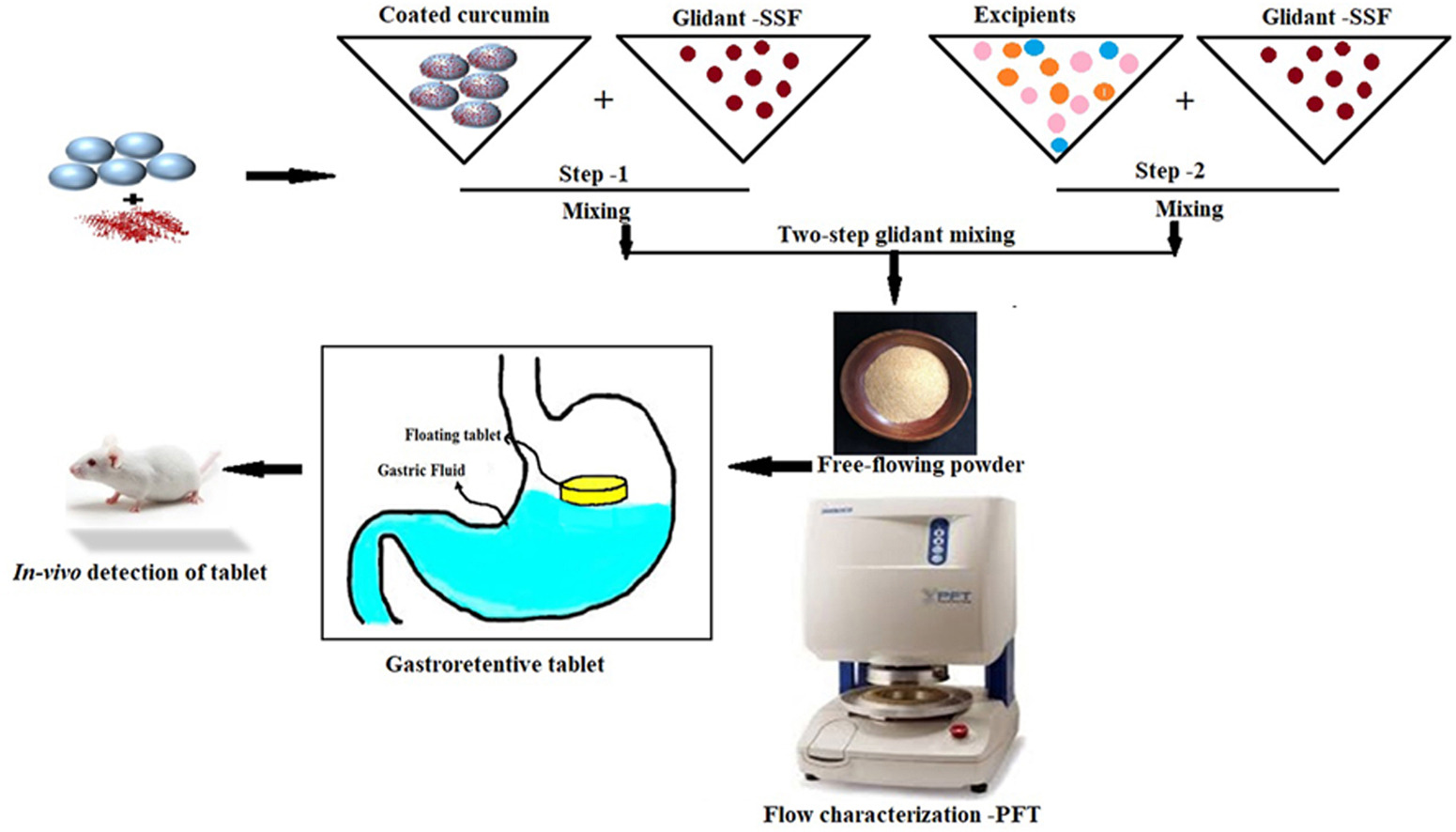
Curcumin is a well-known phenolic compound obtained from Curcuma Longa L. It is used popularly as an antioxidant, anti-inflammatory, antispasmodic, antithrombotic, anticancer as well as immune-modulator. In the last couple of years, few studies showed the usefulness of curcumin against Helicobacter pylori along with the potential to restore gastric damage. However, limited solubility, poor alkaline pH stability and flow property have rendered the industrial application of curcumin. The current research focuses to address this limitation by modifying the flow property using coating and two-step glidant mixing process and then converted into dosage form.
Highlights:
- Targeted limited solubility, poor alkaline pH stability and poor flow property of curcumin which rendered the industrial application of curcumin
- Modified the flow property of curcumin using coating and a two-step glidant mixing process and then converted it into dosage form
- Investigated the flow property by reproducible and reliable advanced method – Powder flow tester (PFT).
- Follow-up study of previously published work on glidant optimization and development of gastro retentive tablet in JDDST.
- Detailed post-compression evaluation –in-vitro, in-vivo and shelf life study.
Furthermore; to have reproducible and reliable results, flow property was investigated by the advance methodology- powder flow tester (PFT). Initially, curcumin powder was coated with HPMC and further mixed with glidant Sodium Stearyl Fumarate by two methods viz. one-step and two-steps mixing operation. Both blends were investigated for various parameters viz. flow function test, wall friction test and bulk density. We found that a two-step glidant mixing operation to coated curcumin enhanced powder to flow significantly more than one-step.
In the lateral stage, both blends were converted into gastroretentive tablets by using the direct compression method. Tablets prepared by using a two-step blend process showed more satisfactory results than one-steps with floating time of 24 h and 21 h respectively. Coating, two-step glidant mixing and PFT were found unique combined approach to prepare the direct compression tablet of curcumin, as it enables the industry to overcome production problems and ensure high-quality products.
Download the full article as PDF here Impact of two-step glidant mixing process on flow performance of coated curcumin – in vitro, in vivo investigation
or read it here
Materials
Sigma Aldrich, Mumbai, India, gifted curcumin and piperine as a free sample for purely research purposes. Hydroxypropyl methylcelluloseK15M (HPMC K15M) and sodium bicarbonate were provided by Colorcon Asia Pvt. Ltd, ltd. Goa, India, and JRS Pharma, Rosenberg, Germany respectively. As a gratis sample from Signet chemical corporation Pvt, Ltd, Mumbai, India, sodium stearyl fumarate (SSF) was given. Microcrystalline cellulose as well as Maltodextrin DE12 was received from Hi-Media Laboratories Pvt. Ltd. Bombay, India as gift samples.
Umesh D. Laddha, Nikhil Girase, Bhushan A. Bhairav, Vijay Lonkar, Shailesh S. Chalikwar, Kailas K. Moravkar,
Impact of two-step glidant mixing process on flow performance of coated curcumin – in vitro, in vivo investigation,
European Journal of Medicinal Chemistry Reports, 2024, 100163, ISSN 2772-4174, https://doi.org/10.1016/j.ejmcr.2024.100163.


