High purity excipients – A Simple Solution to a Complex Problem
A drug-dev.com article by Dr. William Small and Arsalan Khan
It wouldn’t come as a surprise to anyone that active pharmaceutical ingredients (APIs) come in a wide variety. Small molecule, large molecule, peptide, monoclonal antibody, innovative, generic; the list goes on. These molecules have the ability to cure or mitigate debilitating conditions that can change a person’s life forever. It makes sense, then, that these ingredients are of primary importance in a formulation, and appropriate measures should be taken to maintain their stability and efficacy. As these APIs become more complex, they also become increasingly vulnerable to a series of different degradation pathways.
Changes in pH environments can cause acidification and lead to breakdown. Exposure to moisture can initiate hydrolysis and subsequently lead to the formation of secondary byproducts. Residual catalyst that isn’t removed from an excipient can trigger side reactions and perpetuate degradation of not just the API, but everything else in the formulation. To combat this, formulators will typically front-load their formulations to compensate for this anticipated loss.
However, this does not end up being a practical solution, as the degradants are still forming, and becomes an even bigger concern when the cost of developing the formulation becomes even higher. As a result, the more practical solution is to ensure that the remaining ingredients in the formulation are of the highest quality and purity. This certifies the drug will not degrade, and that efficacy and longevity are maintained.
IMPROVED DOCETAXEL RECOVERY VIA HIGH PURITY
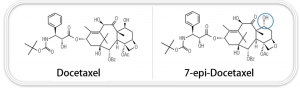
Docetaxel is a great example of where the importance of purity plays a meaningful role. This active, a member of the taxanes class of molecules, is used as a chemotherapy drug, primarily in the treatment of cancers, including breast, lung, prostate, and stomach. Figure 1 depicts the main degradation product for docetaxel, 7-epi-docetaxel. With the same molecular weight as docetaxel, 7-epi-docetaxel is an epimer – a structural stereoisomer with the hydroxyl group at the C7 position (“flipping” position).
Literature on the stability of the taxanes suggests that this is a common degradation product for docetaxel at that site, either through a retro aldol reaction or formation of an enolate intermediate. The formation of 7-epi-docetaxel has been observed in basic and strongly acidic conditions and in the presence of electrophilic agents, though the epimerization can be inhibited in the presence of a metal salt. 7-epi- docetaxel has been found to be less cytotoxic to leukemia cells compared to docetaxel, so the formation of this epimer could reduce the efficacy of the treatment
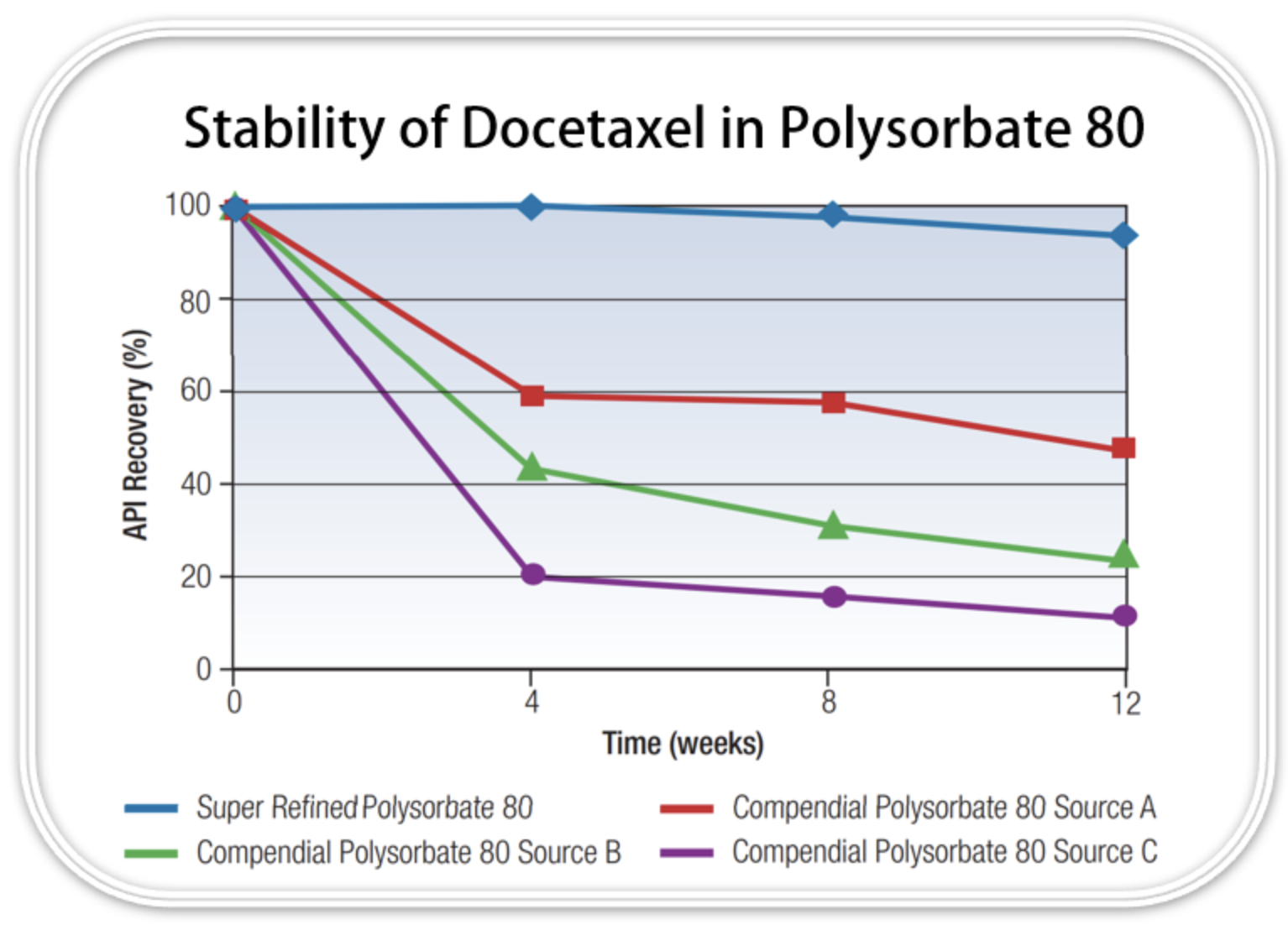
A study conducted on docetaxel comparing its stability in various grades of polysorbate 80 (Figure 2) showed that there is significantly improved (up to 80% higher) recovery after 12 weeks at 40°C, when using a high purity grade rather than a standard compendial grade. Additionally, the study showed that there is a much higher concentration of docetaxel degradants, including 7-epi-docetaxel, present after these same conditions when using a standard compendial grade. This enhanced profile of docetaxel when using a higher purity grade of polysorbate 80, both during standard and accelerated conditions, shows that there are significant benefits from selecting the right grade of excipient when formulating. Continue reading the drug-dev.com article here

