Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation
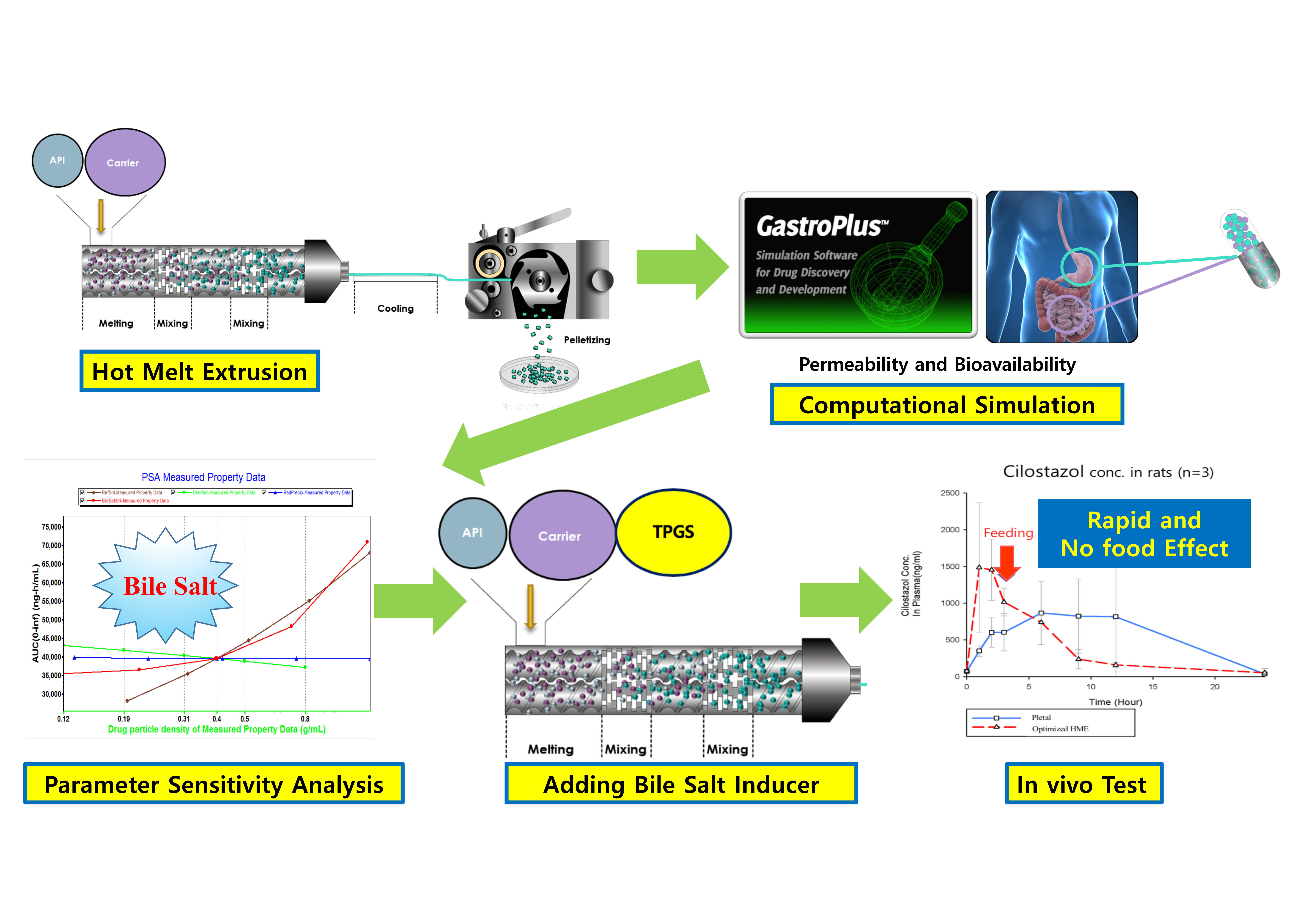
The aim of this study was to control the dissolution rate and permeability of cilostazol. To enhance the dissolution rate of the active pharmaceutical ingredient (API), hot-melt extrusion (HME) technology was applied to prepare a solid dispersion (SD). To control permeability in the gastrointestinal tract regardless of food intake, the HME process was optimized based on physiologically based pharmacokinetic (PBPK) simulation. The extrudates were produced using a laboratory-scale twin-screw hot-melt extruder with co-rotatory screws and a constant feeding rate.
Next, for PBPK simulation, parameter-sensitive analysis (PSA) was conducted to determine the optimization approach direction. As demonstrated by the dissolution test, the solubility of extrudate was enhanced comparing cilostazol alone. Based on the PSA analysis, the surfactant induction was a crucial factor in cilostazol absorption; thus, an extrudate with an even distribution of lipids was produced using hot-melt extrusion technology, for inducing the bile salts in the gastrointestinal tract. In vivo experiments with rats demonstrated that the optimized hot-melt extruded formulation was absorbed more rapidly with lower deviation and regardless of the meal consumed when compared to marketed cilostazol formulations.
Download full paper as PDF: Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption
Choi, S.-M.; Lee, S.-H.; Kang, C.-Y.; Park, J.-B. Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation. Pharmaceutics 2020, 12, 757.
https://doi.org/10.3390/pharmaceutics12080757
Used Excipients: KollidonVA64, Vitamin E TPGS, Microcrystalline Cellulose (MCC)

