Hot-melt extruded lipidic pellets for pediatric applications: An investigation of the effects and stability on drug dissolution
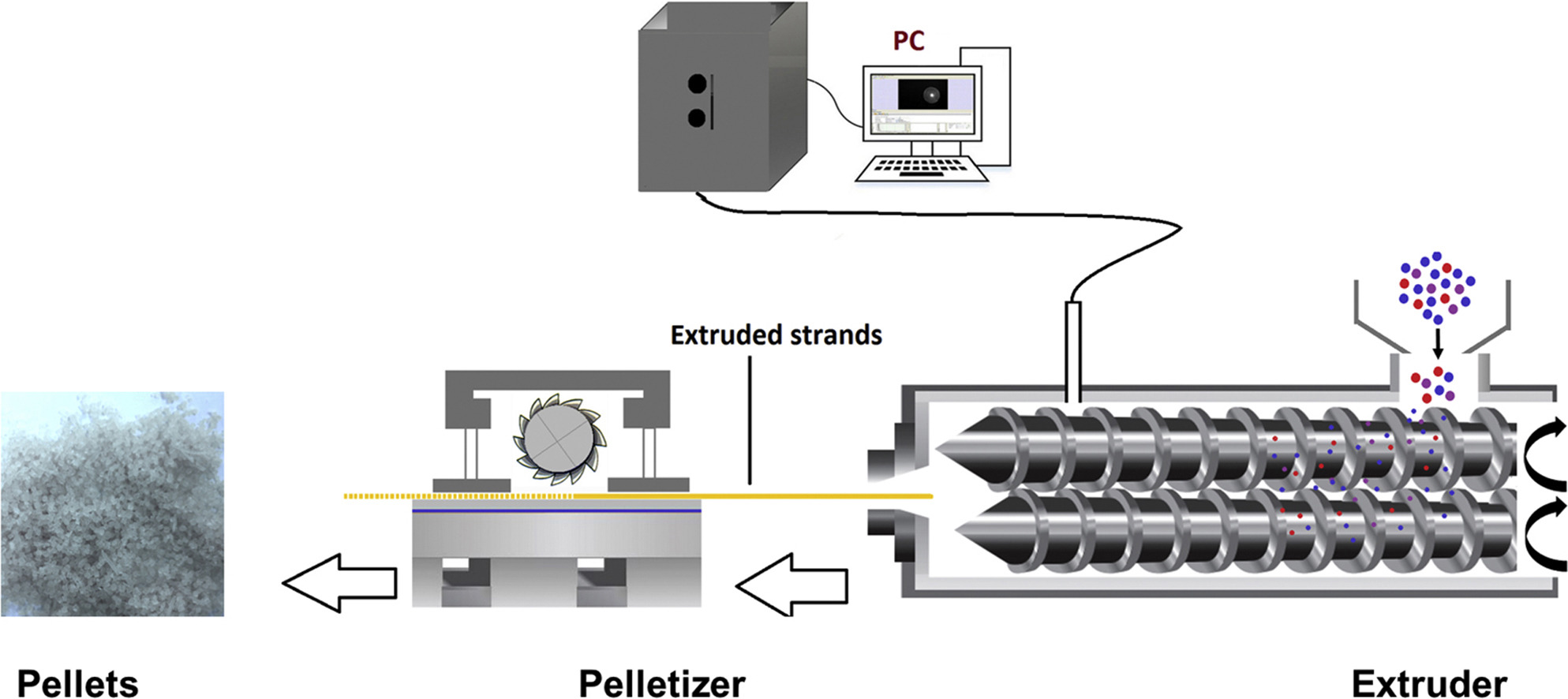
The current study investigates the development of extemporaneous preparation of hot melt extruded diclofenac sodium (Df-Na) pellets processed with glyceryl dibehenate (Compritol® 888 ATO) and dibasic calcium phosphate anhydrous (Fujicalin®) as carriers for pediatric applications.
The extruded lipidic pellets were physicochemically characterized in order to examine the state of the active substance within the extruded formulations where Df-Na was found to retain its crystallinity but partially solubilized by glyceryl dibehenate. Furthermore, the dissolution rates of extemporaneous Df-Na pellets were assessed at gastric (pH 1.1 and simulated gastric fluid pH 1.6) and intestinal conditions (pH 5.5) to investigate the effect of food grades such as yogurt, apple sauce, mashed potato and hypromellose 2910.
Extemporaneous compositions appeared to have a significant effect on Df-Na dissolution rates, especially in presence of apple sauce compared to the other food types. Furthermore, storage under long-term and accelerated conditions showed an effect on drug release from pellets. More on lipid pellets for pediatric applications

