Evaluating human milk as a drug delivery vehicle for clofazimine to premature infants
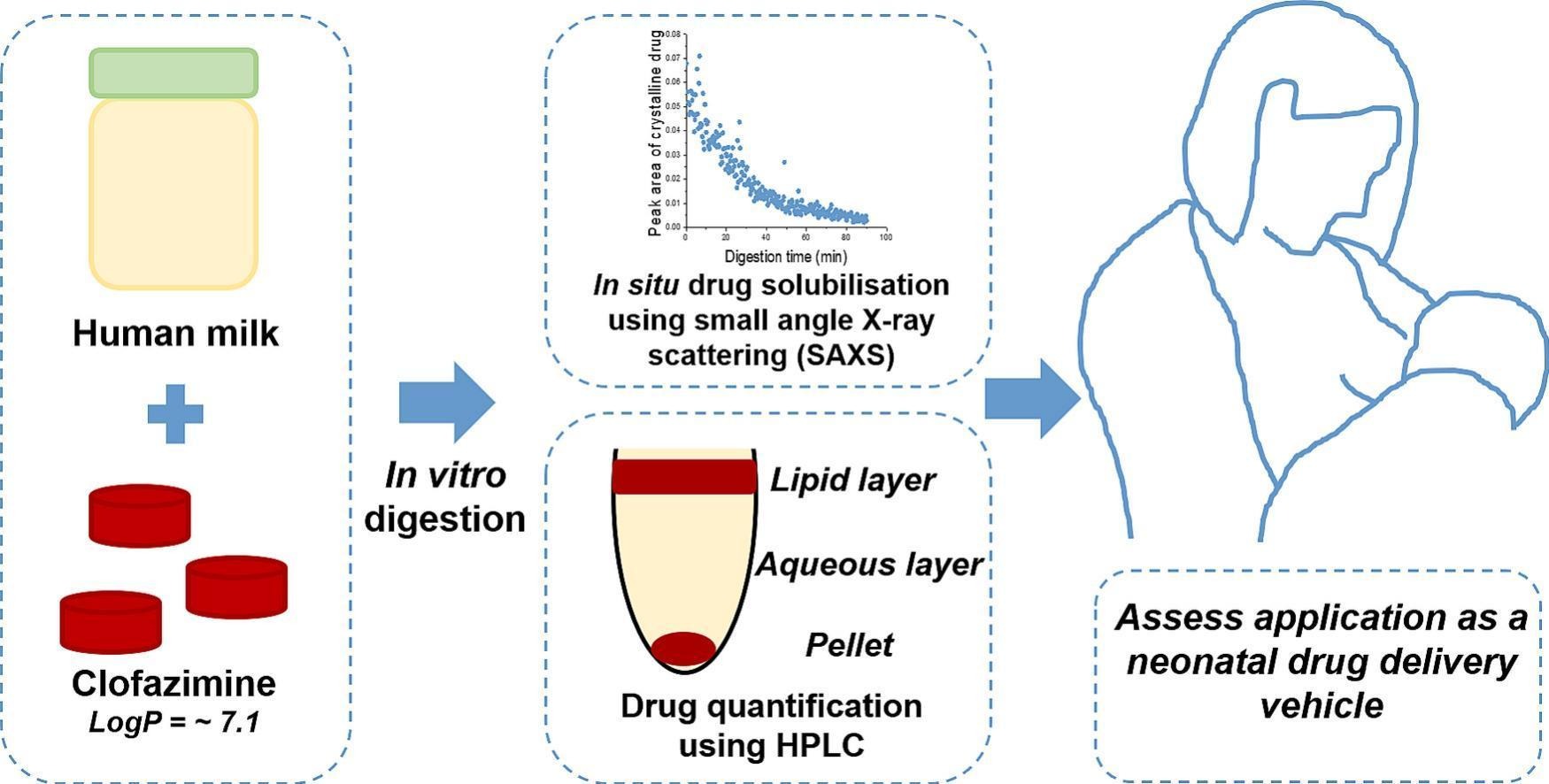
Human milk is proposed as a drug delivery vehicle suitable for use in neonatal patients. Clofazimine, traditionally used for the treatment of leprosy and tuberculosis, is emerging as a treatment for cryptosporidiosis in infants, however its poor aqueous solubility has led to its commercial formulation as a waxy lipid formulation in a capsule, a format that is not suitable for infants. In this study, the evaluation of pasteurised human milk for the delivery of clofazimine was investigated using an in vitro lipolysis model to simulate gastric and intestinal digestion. The total lipid composition of the human milk was characterised alongside the liberated fatty acid species following digestion for comparison to alternative lipid-based delivery systems. Small-angle X-ray scattering was used to measure the presence of crystalline clofazimine during digestion and hence the extent of drug solubilisation. High-performance liquid chromatography was used to quantify the mass of clofazimine solubilised per gram of human milk fat (drug-to-fat ratio) in digested and undigested human milk. The digestion process was essential for the solubilisation of clofazimine, with digested human milk solubilising a sufficient dose of clofazimine for treatment of a premature infant. Human milk solubilised the clofazimine to a greater extent than bovine milk and infant formula during digestion, most likely as a result of differing lipid composition and increased long-chain fatty acid concentrations. These findings show that human milk enhances the solubility of clofazimine as a model drug and may be a suitable drug delivery vehicle for infant populations requiring therapeutic treatment.
1. Introduction
Following birth, many neonates who require pharmacological treatments will be admitted to the neonatal intensive care unit. This is especially the case for premature infants who are more likely to develop complications such as respiratory distress syndrome or necrotizing enterocolitis (NEC) as a result of their immature physiological development [1].
Drug delivery to neonates presents a challenge due to the lack of age-appropriate formulations, which is especially problematic for coadministration of different compounds. The oral delivery of medicines is preferred, with the European Medicines Agency suggesting the use of oral liquid preparations (solutions, suspensions and droplets) as appropriate drug delivery forms following birth [1]. Although oral delivery is a less invasive delivery option than intravenous administration, the lack of appropriate neonatal formulations often results in high occurrences of off-label oral drug use, with up to half the medicines used in the United States not providing adequate labelling for use in children [2]. Off-label use in infants poses a large safety risk as neonates and adults exhibit vastly different stages of development and physiology, resulting in differences in safety, dosing and efficacy requirements [3]. Additionally, the low volumes of fluids (10–20 mL/h) that can be safely tolerated orally by the neonates is an additional factor that must be considered when delivering therapeutics to this vulnerable population [4].
Consequently, parenteral intravenous administration is often used despite a lack of accurate dosing information and limited pharmacokinetic and pharmacodynamic data in infants. Administration via the parenteral route in neonates exhibits many challenges, including being a more invasive and demanding process, requiring a trained health professional and the increased risk of infection and pain at the administration site [5].
Orally delivered lipid-based formulations often facilitate the improved gastrointestinal solubility of poorly water-soluble drugs by the generation of lipophilic colloidal domains [6], however, commercial lipid-based formulations often contain excipients such as surfactants and solvents that are unacceptable for use in neonates. In recent years, the use of milk-related systems such as infant formula, has been of interest as low-cost drug delivery vehicles for paediatric populations [[7], [8], [9], [10], [11], [12]]. Specifically, the co-administration of drug dispersed in reconstituted powdered milk or infant formula provides benefits from a drug stability perspective and amenable to use in harsh climatic conditions compared to liquid formulations. The delivery mechanism relies on digestion of the lipids in the gastrointestinal tract to form fatty acids and monoglycerides that act to provide a dissolution sink for the drug, maximising its solubility and providing a means to enhance bioavailability.
Human milk is recommended as the ideal source of nutrition for infants in the first six months of life [13]. Additionally, the use of an exclusively human milk diet has been shown to potentially decrease the risk of NEC when compared to a diet containing bovine milk-based products such as infant formula and breast milk fortifiers [[14], [15], [16]]. Therefore, from a safety perspective, its use is envisaged to be favoured over infant formula as a drug delivery excipient. Although the use of human milk has been proposed previously as a means of dissolving solid dose forms to enable delivery during breast feeding [[17], [18], [19]], the solubilisation of drugs in human milk during digestion has not been assessed.
To investigate the suitability of human milk as a drug delivery vehicle for neonates, the solubilisation of clofazimine, a highly lipophilic poorly water-soluble drug (log P = 7.1) [20] was selected. As a biopharmaceutical class (BSC) II drug, the dissolution of clofazimine can often be the rate limiting step for oral absorption in the gastrointestinal tract. Clofazimine is currently used in an off-label compounded suspension form in children for tuberculosis and also is emerging as a treatment for crytosporidiosis, a conditon that can result in severe diarrhoea and even death in children [21,22]. Clofazimine has been previously shown to be solubilised during the digestion of milk-based formulations in vitro [9]. Dosing of clofazimine following a high-fat meal has also been shown to improve bioavailability compared to fasted conditions suggesting administration within a suspension of human milk may show similar enhancements [23].
In this study, as illustrated schematically in Fig. 1, the solubility of clofazimine in human milk and the solubilisation behaviour of clofazimine in digesting human milk were measured under gastric and intestinal in vitro conditions. Synchrotron small-angle X-ray scattering (SAXS) was used to monitor the presence of crystalline drug in situ during digestion and used as an indirect measurement of the solubilisation of clofazimine in the digesting milk. The lipid and micronutrient content of the human milk used in the solubilisation studies were characterised using gas chromatography with flame ionisation detector (GC-FID) and inductively coupled plasma-optical emission spectroscopy (ICP-OES), respectively, as these components are known to vary across postpartum stage, duration of feeding, nutritional status and maternal diet [24,25]. The influence of the lipid composition on the solubilisation of clofazimine was compared to the solubilisation behaviour of clofazimine in alternative milk-based formulations used in neonatal populations, namely infant formula and bovine milk [9].
Download the full study as PDF here: Evaluating human milk as a drug delivery vehicle for clofazimine to premature infants
or read it here
Ellie Ponsonby-Thomas, Malinda Salim, Laura D. Klein, Andrew J. Clulow, Susi Seibt, Ben J. Boyd, Evaluating human milk as a drug delivery vehicle for clofazimine to premature infants, Journal of Controlled Release, Volume 362, 2023, Pages 257-267, ISSN 0168-3659,
https://doi.org/10.1016/j.jconrel.2023.08.037.

