Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs
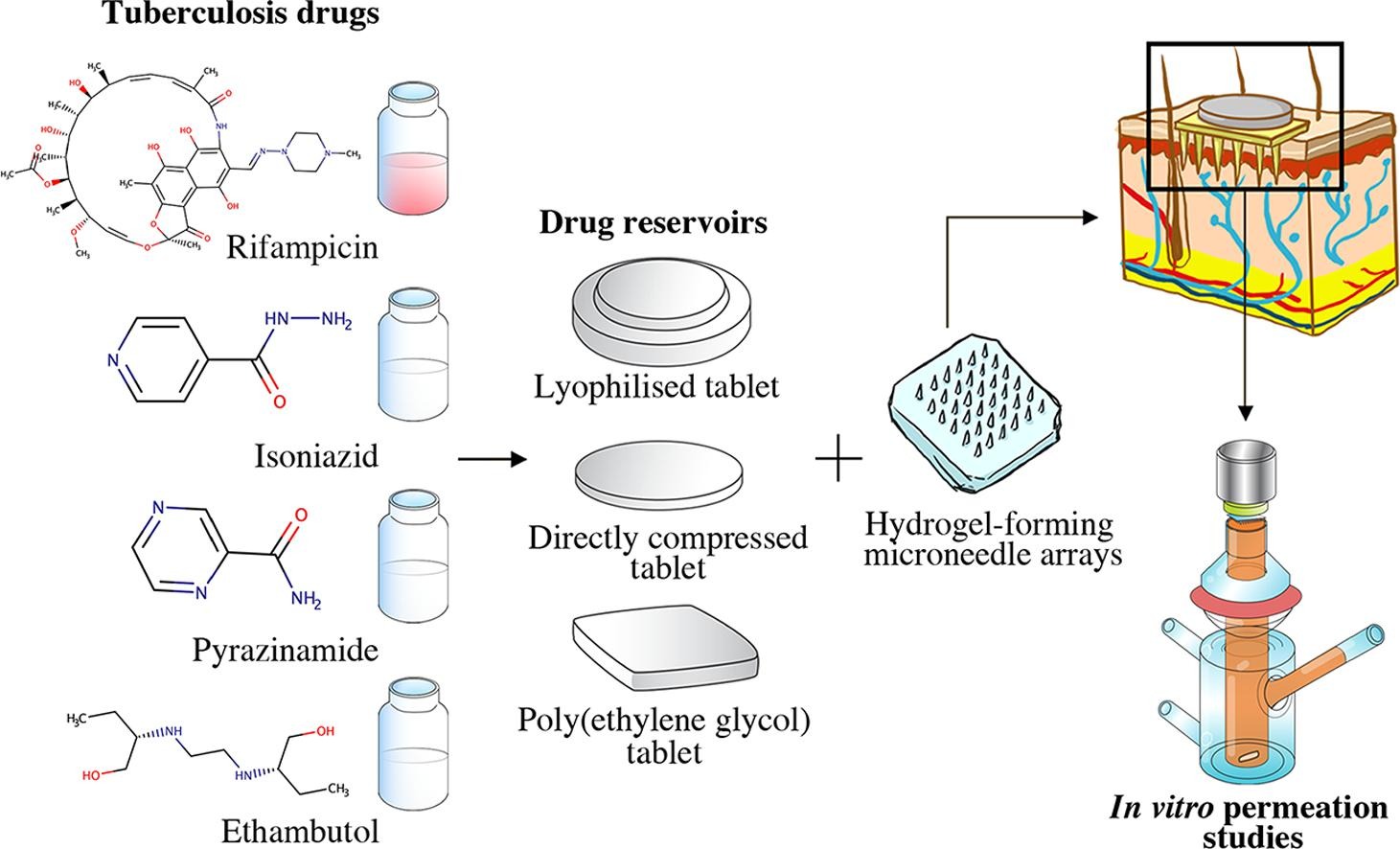
Current therapy of tuberculosis (TB) has several limitations, such as risk of liver injury and intestinal dysbiosis due to frequent oral administration of antibiotics. Transdermal administration could be used to improve antibiotic delivery for treatment of Mycobacterium tuberculosis infection. Therefore, we developed a novel approach, using hydrogel-forming microneedle (MN) arrays to transdermally deliver TB drugs, namely rifampicin, isoniazid, pyrazinamide and ethambutol, which have different physicochemical properties. These drugs were individually prepared into three types of drug reservoirs, including lyophilised tablets, directly compressed tablets and poly(ethylene glycol) tablets. Formulations of each drug reservoir type were optimised to achieve a rapidly dissolving tablet, and further integrated with hydrogel-forming MN arrays for in vitro permeation studies. Three types of hydrogel formulation were manufactured using different type of polymers and crosslinking processes.
These MN arrays were then evaluated in terms of swelling ability, morphology and physical properties. Results of solute diffusion studies showed that drug permeation across the swollen hydrogel membrane was affected mostly by physiochemical properties and functional groups of each drug. In the in vitro studies, the amount of permeated drug through the hydrogel-forming MN arrays across the dermatomed neonatal porcine skin was affected by the drug solubility and reservoir design. The highest permeation of rifampicin (3.64 mg) and ethambutol (46.99 mg) were achieved using MN arrays combined with the poly(ethylene glycol) tablets and directly compressed tablet, respectively. For isoniazid and pyrazinamide, the highest drug permeation was attained using lyophilised reservoir with the amount of drug delivered approximately 58.45 mg and 20.08 mg, respectively. These equate to transdermal delivery of approximately 75% (rifampicin), 79% (isoniazid), 20% (pyrazinamide) and 47% (ethambutol) of the drugs loaded into the reservoirs on average. Importantly, the results of this work have demonstrated the versatility of hydrogel formulations to deliver a TB drug regime using MN arrays. Accordingly, this is a promising approach to deliver high dose of TB drugs.
Qonita Kurnia Anjani, Andi Dian Permana, Álvaro Cárcamo-Martínez, Juan Domínguez-Robles, Ismaiel A. Tekko, Eneko Larrañeta, Lalit K. Vora, Delly Ramadon, Ryan F. Donnelly,
Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs,
European Journal of Pharmaceutics and Biopharmaceutics,
Volume 158, 2021, Pages 294-312,
https://doi.org/10.1016/j.ejpb.2020.12.003.

