Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules
Abstract
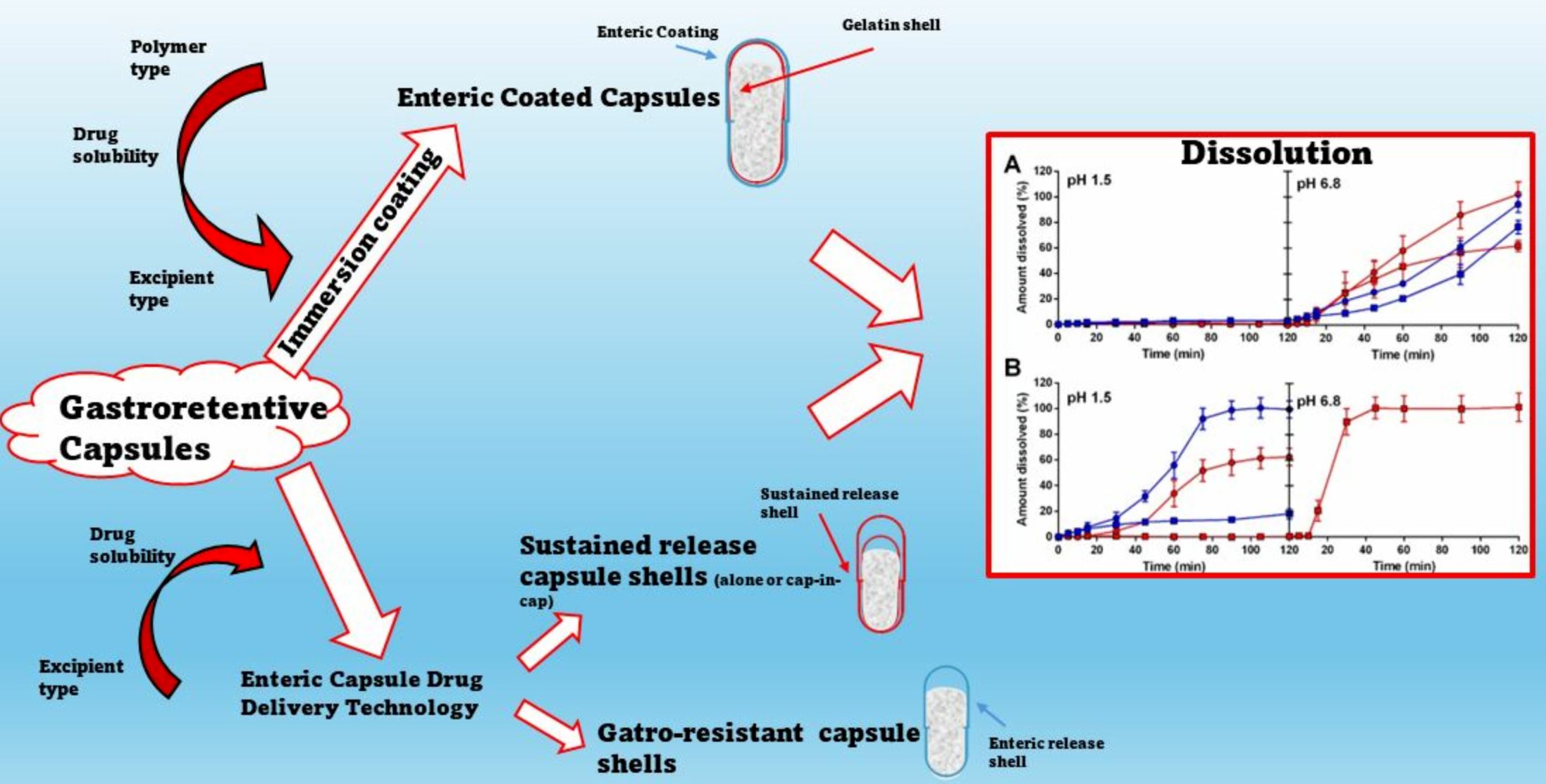
Introduction
or read it here
Materials
Paracetamol and tramadol HCl (both supplied by Janssen Pharma) were chosen as model drugs. Methacrylic acid–methyl methacrylate copolymers (Eudragit S100 and L100) were from Evonik Industries (Essen, Germany). Hypromellose acetate succinate (HPMCAS) (MF and HF grades) were obtained from Shin-Etsu Chemical (Tokyo, Japan). Silicified microcrystalline cellulose (Prosolv sMCC90, JRS Pharma, Rosenberg, Germany), anhydrous lactose (SuperTab 24AN, DFE Pharma, Goch, Germany) anhydrous dibasic calcium phosphate (DI-CAFOS A 60, Budenheim, Budenheim, Germany), and Triethyl Citrate (RoFarma Italia, Gaggiano, Italy) were used as received. Isopropanol (Carlo Erba, Cornaredo, Italy) and Yellow Eosin (Carlo Erba, IT) were of standard chemical grades. Gelatin capsules were purchased from ACEF (Fiorenzuola D’Arda, Italy), while HPMC cps (size 0), DRcaps® (size 2 and size 0), and Vcaps® Enteric capsules (size 0) were obtained from Lonza (Lonza Capsules & Health Ingredients, Basel, Switzerland).
Throughout the text, the following abbreviations are used: paracetamol (AAP), tramadol HCl (Tram), Eudragit S100 (EuS100), Eudragit L100 (EuL100), hypromellose acetate succinate MF (HPMC AS-MF), hypromellose acetate succinate HF (HPMC AS-HF), Triethyl Citrate (TEC), Prosolv sMCC90 (SMCC), SuperTab 24AN (LAC), DI-CAFOS A 60 (DCP), and isopropanol (Iso).
Sabbatini, B.; Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules. Pharmaceuticals 2024, 17, 433. https://doi.org/10.3390/ph17040433
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


