InhaLac® 145 – MEGGLE launches new milled lactose grade
Filling the gap between InhaLac® 140 and InhaLac® 150

Wasserburg, Germany, December, 2023:
Pharmaceutical lactose specialist MEGGLE will use the Drug Delivery to the Lungs (DDL) Conference in Edinburgh, Scotland, to launch InhaLac® 145 as the latest addition to its wide range of lactose-based dry powder inhalation (DPI) excipients.
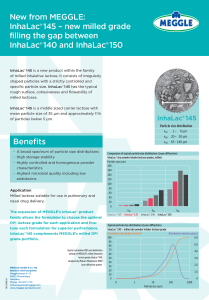
MEGGLE’s InhaLac® 145 is a recent addition to its wide range of InhaLac® high quality crystalline lactose-based excipients specifically designed to support DPI dry powder inhaler formulations. Inhalac® 145 offers DPI drug formulators a highly controlled milled lactose with superior characteristics and highly uniform particle size distribution (PSD).
Meeting DPI challenges
Very specific carrier lactose excipient PSDs are needed to allow formulations to be tailored precisely to target application and extract optimum performance from inhaler devices.
With its mean particle size of 35 μm, the new excipient bridges the small gap between the DPI milled lactose monohydrates InhaLac® 140 (50 μm) and InhaLac® 150 (24 μm).
The InhaLac® 145 PSD profile (x10 = 1-6, x50= 20-50, x90 = 65-140) ensures around 10 per cent of particles below 5μm, retaining the typical flow and surface characteristics of milled lactose with irregular particle shapes.
InhaLac® 145 benefits and quality
These features add up to a clearly defined set of user benefits, including:
– A broad spectrum of particle size distributions
– High storage stability
– Highly controlled and homogenous powder characteristics
– Highest microbial quality including low endotoxins
InhaLac® 145 has been developed harnessing MEGGLE’s long-established expertise in inhalative lactoses, as one of the pioneers in the DPI field. It is manufactured in Wasserburg in Germany, using some of the most sophisticated lactose processing equipment to be found anywhere in the world.
InhaLac® 145 is produced to the highest regulatory and quality standards and in conformance with international pharmacopeias (US-NF, Ph.Eur, JP, ChP).
About MEGGLE
MEGGLE Group GmbH with its headquarter in the Bavarian city of Wasserburg (Germany), operates as a foundation for various business activities in the dairy industry and whey processing. MEGGLE has a proud history of more than 135 years in the dairy market.
The Business Unit Excipients produces pharmaceutical excipients for direct compression, granulation, capsules, sachets, powder blends and dry powder inhalers. With its broad product portfolio, intelligent innovations, and exceptional product quality, MEGGLE plays a leading role in the global pharmaceutical excipients business.
With production facilities in Wasserburg (Germany) and Le Sueur, Minnesota (USA), where the requirements of the North American market are served, MEGGLE is prepared to meet customer expectations worldwide.
See the InhaLac® 145 press release: Press Release_InhaLac 145
See also our interview with Dr. Constanze Müller on the release of InhaLac® 145
See the InhaLac® 145 product flyer here:
(click picture to open the document)