Intranasal drug delivery: opportunities and toxicologic challenges during drug development
Abstract
Over the past 10 years, the interest in intranasal drug delivery in pharmaceutical R&D has increased. This review article summarises information on intranasal administration for local and systemic delivery, as well as for CNS indications. Nasal delivery offers many advantages over standard systemic delivery systems, such as its non-invasive character, a fast onset of action and in many cases reduced side effects due to a more targeted delivery.
There are still formulation limitations and toxicological aspects to be optimised. Intranasal drug delivery in the field of drug development is an interesting delivery route for the treatment of neurological disorders. Systemic approaches often fail to efficiently supply the CNS with drugs.
This review paper describes the anatomical, histological and physiological basis and summarises currently approved drugs for administration via intranasal delivery. Further, the review focuses on toxicological considerations of intranasally applied compounds and discusses formulation aspects that need to be considered for drug development.
Download the very interesting full article as a PDF here or read it here
Introduction
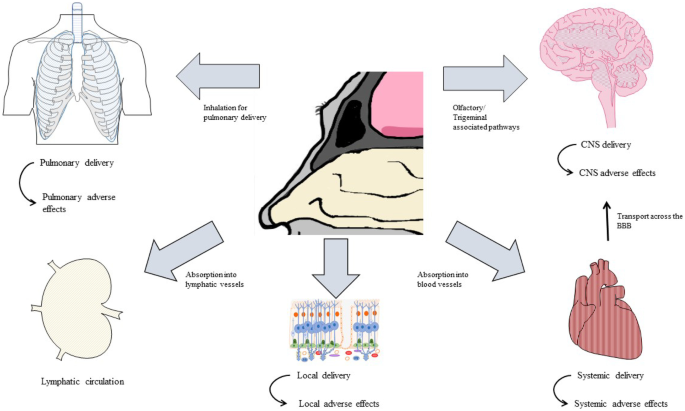
Nasal administration has been used for therapeutic reasons for centuries. As the respiratory tract is a primary contact zone for the environment, it represents a gateway, not only for infectious particles such as bacteria and viruses but also for potential treatments. In the past century, the use of intranasally (IN) administered drugs was mainly restricted to treating topical symptoms of seasonal rhinitis or infectious diseases of the respiratory tract, for example. At the end of the twentieth century, the nasal delivery route became more prominent as an alternative route to treat systemic symptoms such as in cardiovascular indications. The possibility to deliver drugs to the central nervous system (CNS) through nasal pathways remained unexplored until in 1991. William Frey II proposed a patent for a nasal drug delivery method to treat neurological disorders in the brain [1]. Subsequently, there was an increased interest in nasal delivery especially for the growing field of nose-to-brain-delivery (ntb). Ntb delivery, in contrast to systemic delivery, presents a promising alternative enabling the delivery of therapeutic drugs to the CNS, while bypassing the blood-brain barrier (BBB). Compared with conventional drug delivery approaches, ntb delivery represents a non-invasive method, to access the CNS directly through the olfactory or trigeminal nerves. Each drug formulation favours different transport mechanisms, including intracellular and extracellular pathways, from the nasal cavity to the higher brain regions. During drug formulation development, the influence of absorption into the blood circulation, lymphatic systems and into the cerebrospinal fluids has to be considered. For several CNS disorders, specific and efficient therapeutic proteins already exist, and for others, new biologics need to be developed. What they have in common is their potential to improve therapy outcomes and to reduce side effects compared with currently approved systemic medications or compared with the systemic doses currently necessary if delivery to their site of action is enhanced. While there are a number of approved drug formulations for local and systemic indications, the development of nasal drug formulations for CNS delivery is still a challenge. This review paper not only discusses the possibilities and advantages of intranasal drug delivery (INDD) but also highlights its current limitations and toxicological considerations. Anatomical, histological, physiological and pathological information about the nose, together with data on drugs and their formulations, was gathered, to further discuss their influence on INDD and drug development. The future perspectives for the establishment of intranasal drugs for local, systemic and CNS indications are highlighted in this review paper.
Download the full article as a PDF here or read it here
Article information: Keller, LA., Merkel, O. & Popp, A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv. and Transl. Res. (2021). https://doi.org/10.1007/s13346-020-00891-5


