Medication Lubricants for Oral Delivery of Drugs: Oral Processing Reduces Thickness, Changes Characteristics, and Improves Dissolution Profile
Abstract
Introduction
Oral solid dosage forms (i.e., tablets, capsules) are generally designed to be swallowed whole, but many people find this difficult to accomplish. Some people experience a swallowing impairment (dysphagia) as a result of age-related changes in swallowing physiology comorbidities such as stroke, cardiac surgeries, head injury, and polypharmacy [1,2,3]. Food is blended and drinks thickened to produce a consistency that the mouth can safely maneuver into the esophagus rather than the airway [4,5,6], so tablets and capsules can also be unsafe if given whole [7,8]. Individuals with a healthy swallowing function can also struggle to swallow solid oral medications whole [9]. Previous bad experiences with swallowing pills, as well as the physical characteristics of tablets and capsules, i.e., shape, size, and taste, can be factors that lead to this aversion [10].
Using an alternative route of administration or alternative oral formulation, such as liquids or chewable tablets, can be an option [11]. However, the most common way to deal with a whole tablet or capsule that cannot be swallowed whole is to modify it, i.e., crush tablets or open capsules and mix them with food substances or commercial dysphagia-oriented products [9,12]. Around 25% to 33% of medication administration for community-dwelling older patients involved modification of the dosage form to facilitate swallowing [13].
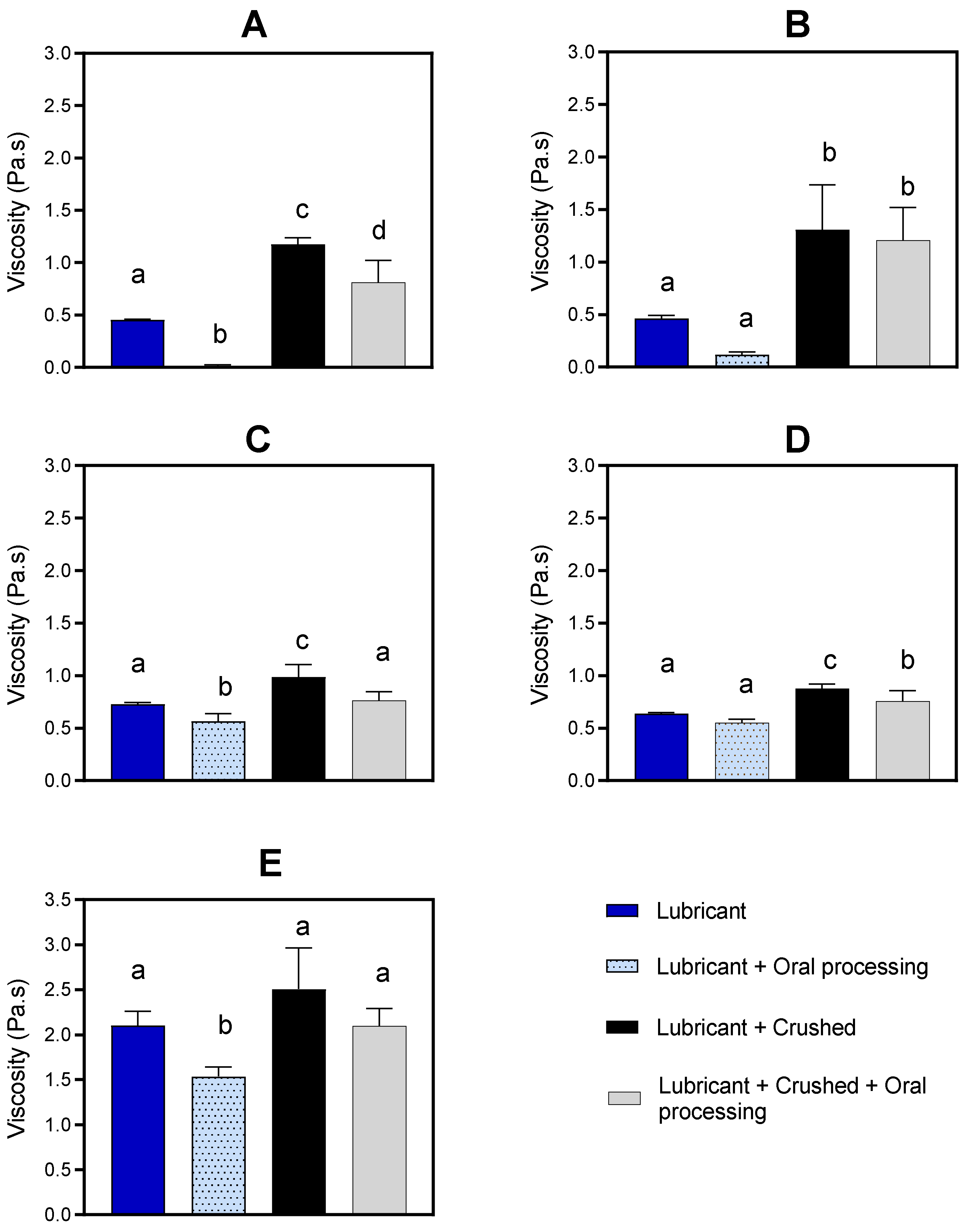
Medication lubricants are a relatively recent product group that has been commercialized. These products were originally designed for use by anyone who struggles to swallow tablets whole using water and who may find that a thicker, lubricating substance better masks the tablet and makes it easier to swallow whole [14]. They are being used to help delivery of both whole and crushed medications. These products are composed of plant gums that form polysaccharide networks. However, it has been demonstrated that mixing crushed medications with dysphagia-oriented thickeners, which are also composed of natural polysaccharide gums that form polymer chain networks, has the potential for a significant delay in disintegration and dissolution [15,16]. For instance, mixing crushed tablets of atenolol with xanthan gum-based thickener showed restriction in drug release in vitro testing, exhibited only 50% release by 30 min, and took 3 h to reach 85% [16]. There is a concern that medication lubricants might impede drug release and therefore impair drug bioavailability.
The first aim of this study is to evaluate the effect of medication lubricants on the drug dissolution of crushed and whole tablets. The in vitro dissolution test is routinely used to provide information about drug-release characteristics and to predict in vivo absorption profiles [17,18]. Additionally, this method has been used in previous research to assess the influence of mixing crushed medications into dysphagia-oriented thickening agents on drug absorption [15,16,19,20]. However, in a previous study, the relationship between in vitro drug release and in vivo drug absorption was not directly aligned.
The in vivo absorption of crushed paracetamol tablets mixed with xanthan gum-based dysphagia-oriented thickeners was substantially faster than what was predicted from in vitro dissolution tests [21]. It has been suggested that this is associated with changes that occur to the bolus structure during deglutition that change the form of the bolus by the time it reaches the stomach, which, in turn, might increase drug release [11,22].
Deglutition is a sequence of voluntary and involuntary reflexes and movements managed by the central nervous system [23]. The oral phase involves various oral operations, including first bite, molding, manipulation, saliva incorporation, mastication, equilibration to the oral cavity temperature, bolus formation, and bolus transportation to the back of the oral cavity to be swallowed. These result in mechanical and physical changes to the bolus structure. The main enzyme in saliva, α-amylase, hydrolyses starch and breaks it down into a simple carbohydrate [24]. Therefore, the mechanical structures of starch-based food and drinks (e.g., custard) are broken down and their viscosity is reduced to less than half within the first 10 s in contact with human saliva [25]. Similarly, the viscosity of starch-based thickeners is affected by deglutition, which consequently threatens swallowing safety for patients with dysphagia [26,27].
Table 1. Details of the five medication swallowing lubricants tested in this study. Flavor and composition details are as provided on the product container; IDDSI classification and yield stress are as reported in [14].
| Name | Manufacturer | Flavour | Composition | IDDSI Classification | Yield Stress (Pa) |
|---|---|---|---|---|---|
| MediSpend | Fagron, Rotterdam, The Netherlands | lemon | Purified water, modified food starch, natural lemon flavour, sodium citrate, citric acid, sucralose, sodium benzoate. | 2—mildly thick | 2.47 |
| Severo | IMS Medical, Grootebroek, The Netherlands | anise | purified water, cellulose gum, flavour (anise), citric acid, potassium sorbate, aspartame, acesulfame K. | 2—mildly thick | - |
| Gloup Low Sugar | Rushwood, Raamsdonksveer, The Netherlands | raspberry | Water, xylitol, carrageenan, maltodextrin, potassium sorbate, citric acid, colour, aroma. | 3—moderately thick | 12.50 |
| Gloup Original | Rushwood, Raamsdonksveer, The Netherlands | strawberry/banana | Water, carrageenan, maltodextrin, potassium sorbate, sucrose, calcium chloride, citric acid, colour, aroma. | 3—moderately thick | 9.76 |
| Gloup Forte | Rushwood, Raamsdonksveer, The Netherlands | vanilla | Water, dried glucose syrup, sucrose, carrageenan, maltodextrin, potassium sorbate, citric acid, (natural) aroma | 4—extremely thick | 38.13 |
By analyzing the physical properties of the bolus during oral processing, a significant decrease in hardness and an increase in springiness, adhesiveness, and cohesiveness of cereal bolus after oral manipulation has been observed [28]. Shearing and elongation of the bolus during transportation through the deglutition process are anticipated to be factors leading to deformation [29,30]. The bolus becomes extremely stretched and deformed while it is transported from the oral cavity to the stomach [31].
Therefore, the second aim of this study is to investigate the effect of oral processing on the physical characteristics (viscosity and texture features) of the medication lubricants and whether it affects the dissolution of whole and crushed tablets mixed with medication lubricants. In this study, the in vivo approach is used, which involves the medication lubricant with/without a paracetamol tablet being placed in the mouth of healthy individuals and prepared for swallowing, and just prior to being swallowed the bolus is collected and characterized in terms of viscosity, texture features, and drug dissolution profile.
Download the full article as PDF here: Medication Lubricants for Oral Delivery of Drugs
or read it here
Materials
Immediate-release 500 mg paracetamol tablets (Panamax, Sanofi Aventis Australia, Macquarie Park, NSW, Australia) were used. Paracetamol is classified in the Biopharmaceutical Classification System (BCS) as class III but possesses some attributes of BCS class I [32]. Paracetamol was used in this study because it has a pKa value of 9.4, which ensures that at the range of pHs encountered in physiological conditions, its solubility is pH-independent [33]. Therefore, the very acidic stomach conditions (pH = 1.2) we used in vitro and the slightly acidic oral environment would have minimal impact on paracetamol solubility. Also, paracetamol is the most frequently modified tablet in Australian hospitals and is most frequently reported as difficult to swallow [34,35]. Moreover, paracetamol has a chromophore within the chemical structure that enables detection by UV spectroscopy.
Five commercial medication swallowing lubricants were tested: MediSpend, Severo Swallowing Gel, Gloup Low Sugar, Gloup Original, and Gloup Forte (Table 1). All are ready-to-use products that are placed onto a spoon, and the pill is placed into it before swallowing [14]. All products were tested at room temperature (approximately 24 °C).
Malouh, M.A.; Cichero, J.A.Y.; Sun, Y.; Lau, E.T.L.; Nissen, L.M.; Steadman, K.J. Medication Lubricants for Oral Delivery of Drugs: Oral Processing Reduces Thickness, Changes Characteristics, and Improves Dissolution Profile. Pharmaceutics 2024, 16, 417. https://doi.org/10.3390/pharmaceutics16030417
Read also our introduction article on Lubricants here:


