A Method for the Tensile Strength Prediction of Tablets with Differing Powder Plasticities
Abstract
Tablets are the most commonly used dosage form in the pharmaceutical industry, and their properties such as disintegration, dissolution, and portability are influenced by their strength. However, in industry, the mixing fraction of powders to obtain a tablet compact with sufficient strength is determined based on empirical rules. Therefore, a method for predicting tablet strength based on the properties of a single material is required. The objective of this study was to quantitatively evaluate the relationship between the compression properties and tablet strength of powder mixtures. The compression properties of the powder mixtures with different plasticities were evaluated based on the force-displacement curves obtained from the powder compression tests. Heckel and compression energy analyses were performed to evaluate compression properties. During the compression energy analysis, the ratio of plastic deformation energy to elastic deformation energy (Ep/Ee) was assumed to be the plastic deformability of the powder. The quantitative relationship between the compression properties and tensile strength of the tablets was investigated. Based on the obtained relationship and the compression properties of a single material, a prediction equation was put forward for the compression properties of the powder mixture. Subsequently, a correlation equation for tablet strength was proposed by combining the values of K and Ep/Ee obtained from the Heckel and compression energy analyses, respectively. Finally, by substituting the compression properties of the single material and the mass fraction of the plastic material into the proposed equation, the tablet strength of the powder mixture with different plastic deformabilities was predicted.
Introduction
Powder compression is the molding process for tablets during which pressure is directly applied to the powders. It has been utilized in various industries, including pharmaceuticals, ceramics, metals, batteries, and foods, because of its simplicity and low-cost mass production.1–6) Particle properties such as shape, size, Young’s modulus, and plastic deformability affect compact properties such as tablet strength and void fraction.7–9) Tablet strength is a significant physical attribute because it strongly affects other important tablet properties, such as disintegration, dissolution, and portability. However, in industry, empirical-rule-based trial and error techniques are used to determine the mixing ratio of components, which strongly affects tablet strength. Therefore, a method for predicting tablet strength based on the properties of a single material is required. Tablet strength is related to the bonding properties between particles, such as the average coordination number of particles and plastic deformation. The relationship between the remaining strain and the tablet strength is often considered through numerical analyses using the finite element method.10,11) The compression process induces significant densification of the powder bed owing to particle rearrangement and deformation. High compression pressure increases both the coordination number and contact area between the particles.12) Although the plastic deformability of individual materials has been evaluated, studies focusing on the plastic deformability of mixed powders and quantitative evaluation methods are insufficient.
Powder compression involves the following four processes: packing, compression, decompression, and ejection.13–17) Because tablet strength is affected by the coordination number and contact area between particles during compression, powder compression behavior analysis is important.18,19) A method for predicting the capping tendency was proposed based on the ratio of elastic to plastic energy obtained from compression energy analysis.20) Measurement of the die wall pressure was also useful for estimating the capping tendency.21,22) Four elementary processes occur simultaneously during powder compaction: rearrangement, elastic and plastic deformations, and brittle fracture.23–27) Particle rearrangement is a phenomenon during which compression causes particles to move to a stable position without deformation, thereby increasing their coordination numbers.28) X-ray microtomography of the compression process of the cohesive nanoparticles revealed that the density distribution in the powder bed during packing becomes uniform after compression.29) Particle deformation can be divided into elastic and plastic deformation. During elastic deformation, the particle returns to its initial shape after unloading, whereas plastic deformation is permanent. The deformation of the powder beds and the plasticity depend on the material characteristics. As high pressure is applied to the powder beds, the powder bed undergoes densification through brittle particle fracture caused by the compressive force. These elementary steps occur simultaneously and are influenced by the powder properties.30) In addition, powder properties such as particle shape, size distribution, cohesiveness, Young’s modulus, and plasticity significantly affect the structure of the compacts.31,32)
Particle plasticity strongly affects the compression properties. Several models have been proposed to describe the plastic deformation of particles.33) The plastic stiffness of granules also affects their compression behavior.34) The compressibility descriptors given by the representative compressibility parameters, compressibility analysis, Walker equation, and rearrangement index of Kawakita analysis have a linear relationship with the formulation of the binary mixture.35) Heckel evaluated the plastic deformability of powder beds by focusing on the relationship between relative density and compression pressure.36) Heckel’s analysis is the most popular method for evaluating the plasticity of powders.37,38) Thus, despite the effect of the mixing fraction of the powder on the compression behavior, it was insufficient to predict the mixing fraction to obtain the required tablet strength.
Therefore, the purpose of this study was to quantitatively evaluate the relationship between the compression and tabletability properties of powders composed of different materials, with a particular focus on the particle plastic deformability. The compression properties of the powder mixtures of different materials with different plastic deformabilities were evaluated based on the force–displacement (F–d) curves obtained through powder compression tests. Heckel and compression energy analyses were performed to evaluate compression properties. In addition, the quantitative relationship between the compression properties and tensile strength of the compacts was investigated. Finally, a prediction equation is proposed for the compressibility of mixed powders based on the obtained relationship and the compression properties of a single material.
Download the full article as PDF here A Method for the Tensile Strength Prediction of Tablets with Differing Powder Plasticities
or read it here
Powders
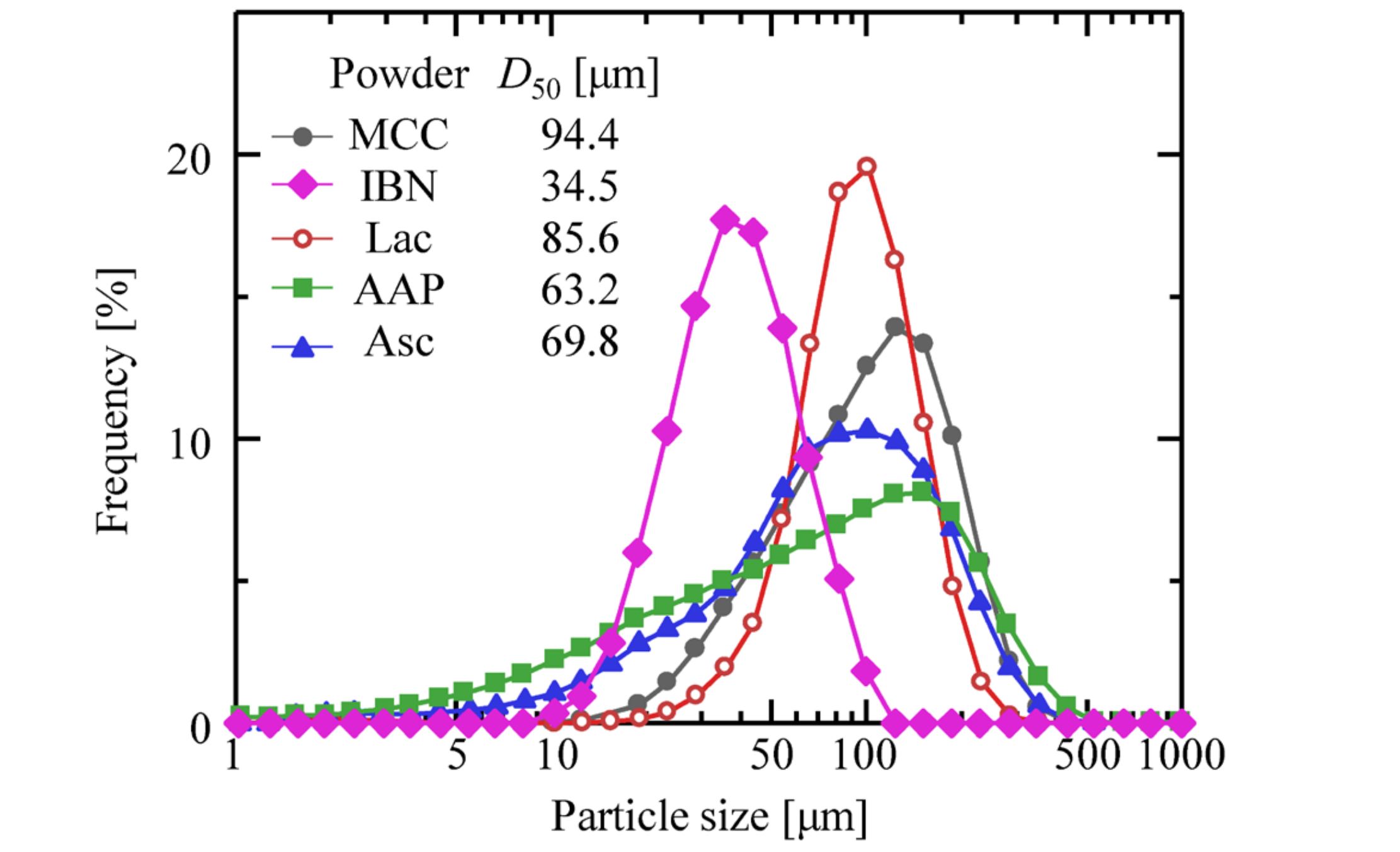
Microcrystalline cellulose (MCC; CEOLUS® PH-101, Asahi Kasei Corp., Japan), ascorbic acid (Asc; L-ascorbic acid, Fuso Chemical Co., Ltd., Japan), acetaminophen (AAP; ACETAMINOPHEN, BASF Japan Ltd., Japan), lactose (Lac; Dylactoze® S, FREUND Corp., Japan), and ibuprofen (IBN; IBUPROFEN, BASF Japan Ltd., Japan) were used. MCC, Lac, and Asc were sieved to sharpen their size distributions, whereas IBN and AAP could not be sieved because of their cohesiveness. The size distributions of the powders were measured under dry conditions using a laser diffraction particle size analyzer (SALD-2100, SHIMADZU Corp., Japan). The size distribution of powders was shown in Fig. 1. The properties of the powder are listed in Table 1. ρtrue and D50 were the true density and median diameter of the particles, respectively.
Takeru Yano, Atsushi Oshiro, Shuji Ohsaki,* Hideya Nakamura, and Satoru Watano, A Method for the Tensile Strength Prediction of Tablets with Differing Powder Plasticities, Received February 6, 2024; accepted March 18, 2024, Chem. Pharm. Bull. 72, 374–380 (2024), Vol. 72, No. 4, https://doi.org/10.1248/cpb.c24-00090
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


