Supercritical fluid technology as a strategy for nifedipine solid dispersions formulation: in vitro and in vivo evaluation
Abstract
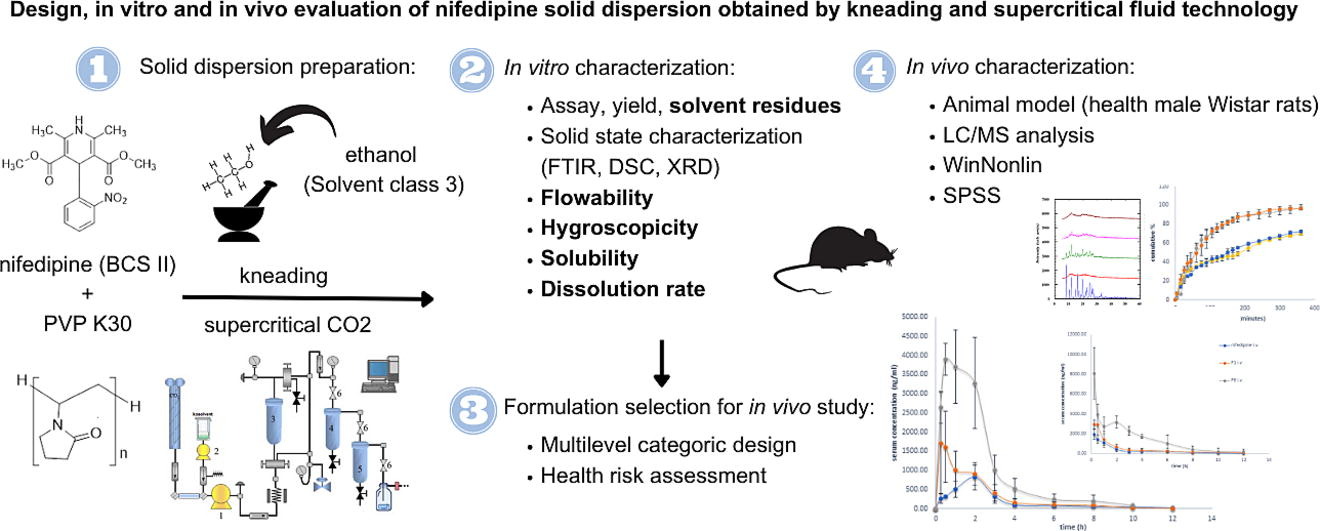
Supercritical fluid technology (SFT) is an insufficiently investigated approach for the production of solid dispersions, it is environmentally acceptable and has a high potential for application in the pharmaceutical industry. The aim of this work was to formulate and characterize nifedipine solid dispersions (SDs) produced by the SFT and compare the results with ones obtained by the classical solvent based kneading method. The following in vitro tests were conducted: assay and yield, solvent residues, solid state characterization (FTIR, DSC, XRD), flowability, hygroscopicity, solubility, dissolution and stability. Additionally, bioavailability was examined on an animal model (Wistar rats). The formulation selection for in vivo study was performed using the multilevel categoric experimental design and the health risk assessment. Solid state characterization revealed that formulation obtained by the SFT method and higher ratio of polymer (1:5) have had nifedipine in completely amorphous form. Polymer ratio and method of SDs preparation do influence the investigation characteristics Dissolution rate was fastest in SDs prepared by the SFT and higher polymer ration (1:5). In vivo data of selected SDs prepared by the kneading (ratio 1:1) and the SFT (ratio 1:5) showed alteration in pharmacokinetic profile after i.v. and p.o. application.
Introduction
Nifedipine (dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate) is a calcium channel blocker with authorized indication for the treatment of hypertension and angina pectoris in adults. According to its biopharmaceutical characteristics (low solubility and high permeability), nifedipine belongs to the Biopharmaceutics Classification System (BCS) class II (Mantas and Mihranyan, 2019, Gajendran et al., 2015). The 11th edition of the European Pharmacopoeia (Ph. Eur. 11) describes nifedipine as a practically water-insoluble substance, which means that more than 10,000 ml of water is necessary to dissolve 1 g of nifedipine (EDQM, 2023). Furthermore, nifedipine solubility at 37 °C is practically consistent at different pH values specific for the gastrointestinal tract: 0.0058 mg/ml at pH 4 and 0.0111 mg/ml at pH 6.8 (Gajendran et al., 2015, Wagner et al., 2013, Boje et al., 1988) which can be attributed to pKa values (pKa1 = − 0.9 and pKa2 = 13) out of the physiological span. Therefore, the changes in the pH value in the physiological range do not affect the solubility of nifedipine (Gajendran et al., 2015, Mannhold et al., 1984). Accordingly, the desirable increment of solubility and dissolution rate of molecules like nifedipine should be achieved by varying other properties such as the formation of non-crystalline molecular structures and/or particle size reduction.
It is estimated that around 40% of medicines on the market contain an active pharmaceutical ingredient (API) with poor solubility. Additionally, up to 90% of new chemical entities have been described with poor solubility which further limits their studies as an API (Takagi et al., 2006, Rodriguez-Aller et al., 2015). Several methods have been developed to increase the solubility of substances. The methodology of solid dispersions (SD) is recognized as one of the most successful, and there are already formulations containing API in the form of a SD on the medicine market. SD are defined as dispersions of water poorly soluble substances in at least one solid carrier. In SD form, the drug surface area, the wettability and the dispersibility are enhanced, while the aggregation and the agglomeration of the drug particles are reduced. Besides, the amorphization of the drugs formulated as the SD was also reported. All abovementioned effects of SD result in increased solubility, dissolution rate and bioavailability. Methods for obtaining SD affect (Schönfeld et al., 2021) their performances and can be divided in two main groups: melting based and solvent based (Tambe et al., 2022). In these processes water soluble polymers (such as poly(vinyl pyrrolidone) (PVP), as drug carrier play an important role.
PVP is one of the most commonly used water soluble, hydrophilic polymers for the production of SD. PVP is a versatile excipient that is generally recognized as a biocompatible, biodegradable, non-antigen, processable and chemically stable substance (Bejaoui et al., 2023, Teodorescu et al., 2019, Smith et al., 2006, Odeh et al., 2023). SD of nifedipine and PVP obtained by different methods such as hot melt extrusion (Ma et al., 2019), dry ball-milling (Saraf et al, 2022), spray drying (Browne et al., 2021, Jung et al., 2022), rotary evaporation (Kim et al., 2022) and simple solvent evaporation method (Maulana et al., 2022) have been investigated.
Supercritical fluid technology (SFT) is an approach in SD formulation with numerous advantages. The SFT is recognized as sustainable, green, safe and ecologically friendly method. In addition, the SFT could be easily implemented in the pharmaceutical industry, since it has been used for years in the food and textile industries and standards for equipment and work processes required by Good Manufacturing Practice (GMP) have already been developed (Milovanović and Lukić, 2022). The application of the SFT overcomes the shortcomings of melting based methods, as well as methods based on organic solvents application. The main advantage of the SFT is that thermostability of API and the polymers, as well as their compatibility and miscibility at high temperatures have no influence on the process should and not be considered when using the SFT. SD obtained by melting based methods can have poor flowability and compressibility, as well as a phase separation may occur during cooling SD obtained by heating (Milovanović and Lukić, 2022, Djuris et al., 2019, Obaidat et al., 2017). Furthermore, the absence of oxygen and overcoming the drying step can be considered as additional advantages of the SFT (Milovanović and Lukić, 2022, Fages et al., 2004, Alsmadi et al., 2022). In comparison to the SFT, kneading method is a simple approach appropriate for applying in the initial steps of SD development. However, the kneading method requires organic solvents, which affect the SD stability and their safety when applied. Residual solvents have been classified into three classes depending on the severity of the risk they pose to human health. Ethanol belongs to class 3 – solvents with low toxic potential and their permitted daily exposure (PDE) has been set at 50 mg/day (EDQM, 2023; ICH, 2021).
Nifedipine is soluble in supercritical carbon dioxide (Knez et al., 1995), thus the SFT is a suitable method for obtaining nifedipine SD. So far, nifedipine has been formulated as SD with PEG 4000 in a mass ratio of 1:4 using the SFT (Senčar-Božić et al., 1997). PVP K30 has been recognized as superior polymer to PEG 4000 for the SFT due to its ability to undergo the experimental conditions required by the SFT and improve the solubility performance of different API from BCS class II such as carvedilol (Djuris et al, 2019) and atorvastatin (Altaani et al., 2020). The SFT was studied as an appropriate method for the development of SD with PVP and different APIs, such as tacrolimus (Obaidat et al, 2017), atorvastatin (Altaani et al., 2020), carvedilol (Djuris et al, 2019), etc.
The aim of the study was to formulate and in vitro characterize nifedipine SDs with PVP K30 as a hydrophilic polymer using the SFT and the kneading method. The influence of the applied preparation method and the polymer ratio on the in vitro characteristics was observed. Furthermore, formulation selection for in vivo characterization on an animal model (Wistar rats) was based on in vitro data, using the multilevel categorical design in DesignExpert software. Health risk assessment associated to ethanol in th SD formulations obtained by the kneading method was being considered. In vivo tests were performed in order to confirm the optimization of the nifedipine pharmacokinetics when it is administered in the form of SDs.
Read more here
Materials
Pharmaceutical grade nifedipine (Ph. Eur. 10.4, Moehs Iberica S.L. Spain) was purchased from Farmalabor (Italy). Poly(vinyl pyrrolidone) K30 (PVP K30, M ≈ 40000 g/mol, Carl Roth GmbH + Co. KG, Germany) was used as hydrophilic polymer to formulate SD. Ethanol, 70% (Livsane, Serbia) was applied as a medium to obtain SD by using the kneading method. Methanol G. R. (lach:ner, Czech Republic) was used to dissolve nifedipine in the assay test.
Nemanja Todorović, Jelena Čanji Panić, Branimir Pavlić, Senka Popović, Ivan Ristić, Srđan Rakić, Ivana Rajšić, Saša Vukmirović, Branislava Srđenović Čonić, Boris Milijašević, Nataša Milošević, Mladena Lalić-Popović,
Supercritical fluid technology as a strategy for nifedipine solid dispersions formulation: in vitro and in vivo evaluation,
International Journal of Pharmaceutics, 2023, 123634, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123634.
Read more on Binder – Pharmaceutical Excipients here:


