Revisiting the Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals
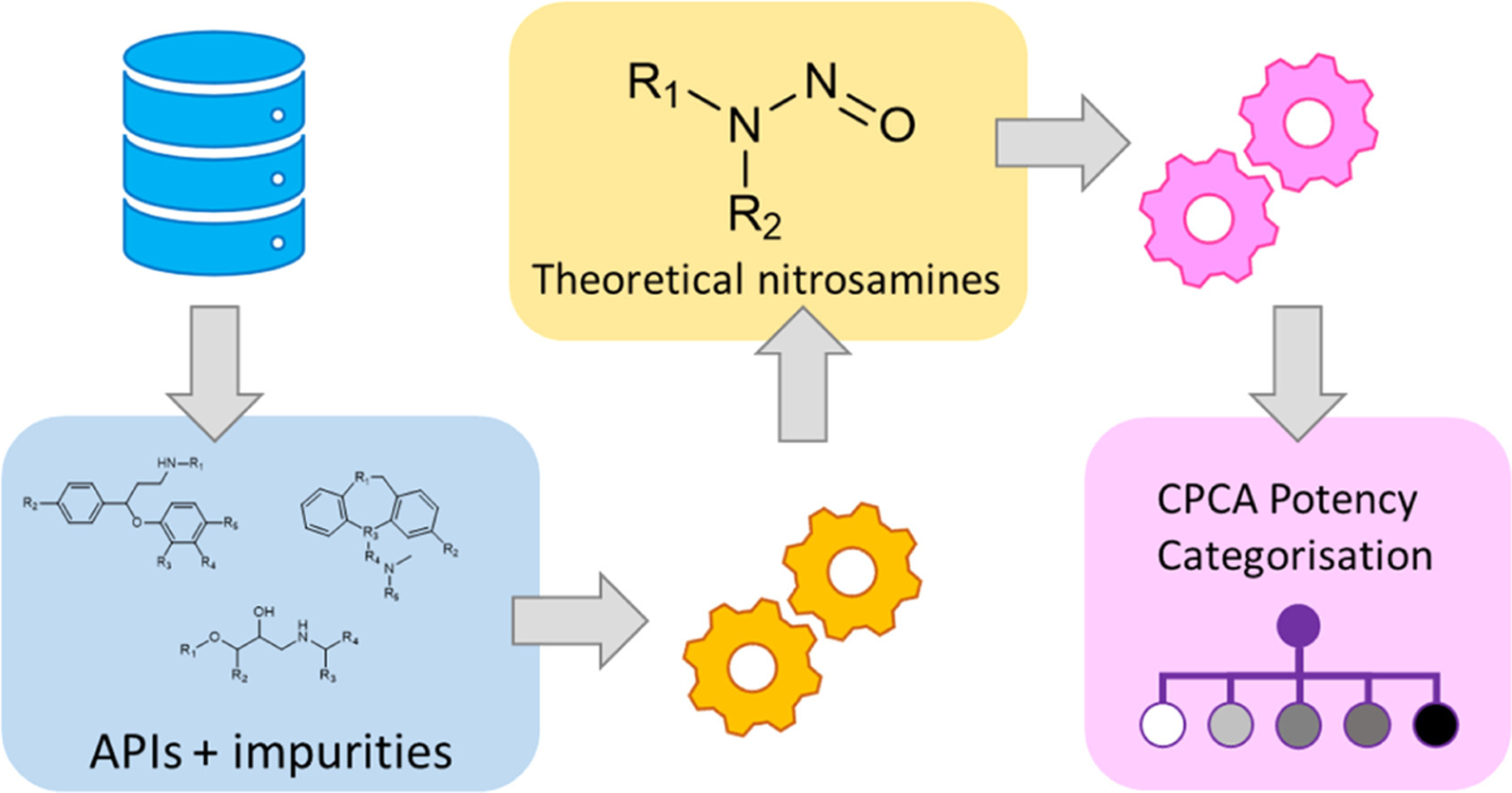
N–Nitrosamines are a class of indirect acting mutagens, as their metabolic degradation leads to the formation of the DNA-alkylating diazonium ion. Following up on the in-silico identification of thousands of nitrosamines that can potentially be derived from small molecule drugs and their known impurities described in a previous publication, we have now re-analyzed this dataset to apply EMA’s Carcinogenic Potency Categorization Approach (CPCA) introduced with the 16th revision of their Q&A document for Marketing Authorization Holders.
We find that the majority of potential nitrosamines from secondary amine precursors belongs to potency categories 4 and 5, corresponding to an acceptable daily intake of 1500 ng, whereas nitrosamines from tertiary amine precursors distribute more evenly among all categories, resulting in a substantial number of structures that are assigned the more challenging acceptable intakes of 18 ng/day and 100 ng/day for potency categories 1 and 2, respectively. However, the nitrosative dealkylation pathway for tertiary amine is generally far slower than the direct nitrosation on secondary amines, with a direct nitrosation mechanism suspected only for structures featuring electron-rich (hetero)aromatic substituents.
This allows for greater focus towards those structures that require further review, and we demonstrate that their number is not substantial. In addition, we reflect on the nitrosamine risk posed by secondary amine API impurities and demonstrate that based on the ICH Q3A/B identification threshold unknown impurities may exist that could be transformed to relevant amounts of NA. We also demonstrate that the analytical sensitivity required for the quantification of high potency nitrosamines can be problematic especially for high dose APIs. In summary, the regulatory framework rolled out with the latest Q&A document represents a substantial improvement compared with the previous situation, but further refinement through interaction between manufacturers, regulators, not-for-profit and academic institutions will be required to ensure patient access to vital medicines without compromising safety.
Download the full study as PDF here: Revisiting the Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals
or read it here
Michael J. Burns, David J. Ponting, Robert S. Foster, Benjamin P. Thornton, Naiffer E. Romero, Graham F. Smith, Ian W. Ashworth, Andrew Teasdale, Stephanie Simon, Joerg Schlingemann, Revisiting the Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals, Journal of Pharmaceutical Sciences, 2023, , ISSN 0022-3549,
https://doi.org/10.1016/j.xphs.2023.10.001.

