Enhancing Oral Delivery of Biologics: A Non-Competitive and Cross-Reactive Anti-Leptin Receptor Nanofitin Demonstrates a Gut-Crossing Capacity in an Ex Vivo Porcine Intestinal Model
Abstract
Biotherapeutics exhibit high efficacy in targeted therapy, but their oral delivery is impeded by the harsh conditions of the gastrointestinal (GI) tract and limited intestinal absorption. This article presents a strategy to overcome the challenges of poor intestinal permeability by using a protein shuttle that specifically binds to an intestinal target, the leptin receptor (LepR), and exploiting its capacity to perform a receptor-mediated transport. Our proof-of-concept study focuses on the characterization and transport of robust affinity proteins, known as Nanofitins, across an ex vivo porcine intestinal model. We describe the potential to deliver biologically active molecules across the mucosa by fusing them with the Nanofitin 1-F08 targeting the LepR. This particular Nanofitin was selected for its absence of competition with leptin, its cross-reactivity with LepR from human, mouse, and pig hosts, and its shuttle capability associated with its ability to induce a receptor-mediated transport. This study paves the way for future in vivo demonstration of a safe and efficient oral-to-systemic delivery of targeted therapies.
Introduction
Recombinant biological molecules, the powerful pioneers of modern medicine, referred to as biologics—encompassing entities such as antibodies, proteins, and peptides—constitute the very backbone of contemporary medical treatments [1,2,3]. However, unlike conventional small-molecule drugs commonly administered orally [4], they are mostly limited to parenteral injections mainly due to their sensitivity to protease degradation and their limited passive diffusion through the intestinal barrier [1]. Specifically, the poor permeability of biologics across the intestinal barrier is attributed to the presence of tight junctions at the border of intestinal epithelial cells which hinder their paracellular transport [5], as well as to the enzymes and transporters expressed by these cells which limit their transcellular passage to specific active mechanisms [6,7,8,9]. While the stability in the GI tract of biologics can be solved by gastro-resistant formulation, enabling the permeability of biologics through the intestinal barrier remains a critical factor in achieving successful oral-to-systemic delivery of targeted biologics-based therapies.
Harnessing the active translocation mechanisms employed by receptors for biomolecule passage through biological barriers has shown promise for drug transport in preclinical studies, particularly across the blood–brain barrier (BBB) that isolates the brain from the bloodstream [10,11,12]. This strategy, known as molecular trojan horse or receptor-mediated transcytosis (RMT), involves targeting various receptors of natural ligands, including the transferrin receptor [13,14], the low-density lipoprotein receptor [14,15], and the insulin receptor [12,16], which can trigger the internalization and transport of their natural ligand across biological barriers. Adopting a similar strategy to facilitate the translocation of biologics across the intestinal barrier could enable their systemic delivery after oral administration.
Several receptors, including the neonatal Fc receptor [17,18] and LepR [19,20], have been partially characterized for their involvement in the active transport of protein-based ligands across the intestinal epithelium, despite the scarcity of information available in this field. The LepR, a member of the class I cytokine receptor family, exists in various isoforms resulting from alternative splicing [21,22] (long, short, or secretory). The membrane-bound isoforms share common extracellular and transmembrane domains, whereas their intracellular regions differ in size and amino-acid sequence. They have an identical affinity for the leptin since the ligand-binding region is located on the shared extracellular segment [23]. Evidence suggests that LepR facilitates a specific, saturable, and energy-dependent process for the transcytosis of leptin across the intestinal barrier [19,24]. Leptin interacts with the extracellular domain of the receptor present on the apical membrane of enterocytes and is internalized by the cells through their endosomal compartments. The leptin-LepR complex is then packaged and discharged on the basolateral membrane to reach the blood circulation across the capillary endothelial cells [24]. Given its biological significance, LepR presents itself as a compelling receptor for RMT.
Our present strategy to enhance the transport of biologics across the intestinal barrier relies on Nanofitins targeting the LepR (anti-LepR Nanofitins), which offer a combination of protein robustness, modularity, and tunable specificity. These small single-chain binding proteins of 7 kDa, devoid of cysteine residues, are derived from the naturally hyper thermostable protein Sac7d [25,26], or more generally from the Sul7d family [27]. They can be custom-engineered for high specificity and affinity toward a target, as already demonstrated against a variety of biological targets [28,29,30,31]. Importantly, they maintain the stability of their parental protein, including resistance to extreme pH levels (pH 1–13) and the harsh intestinal environment [28]. With their N- and C-termini located on opposite faces of their binding site, they can be easily assembled to a cargo molecule, either via chemical conjugation or genetic fusion, while preserving their individual properties [32].
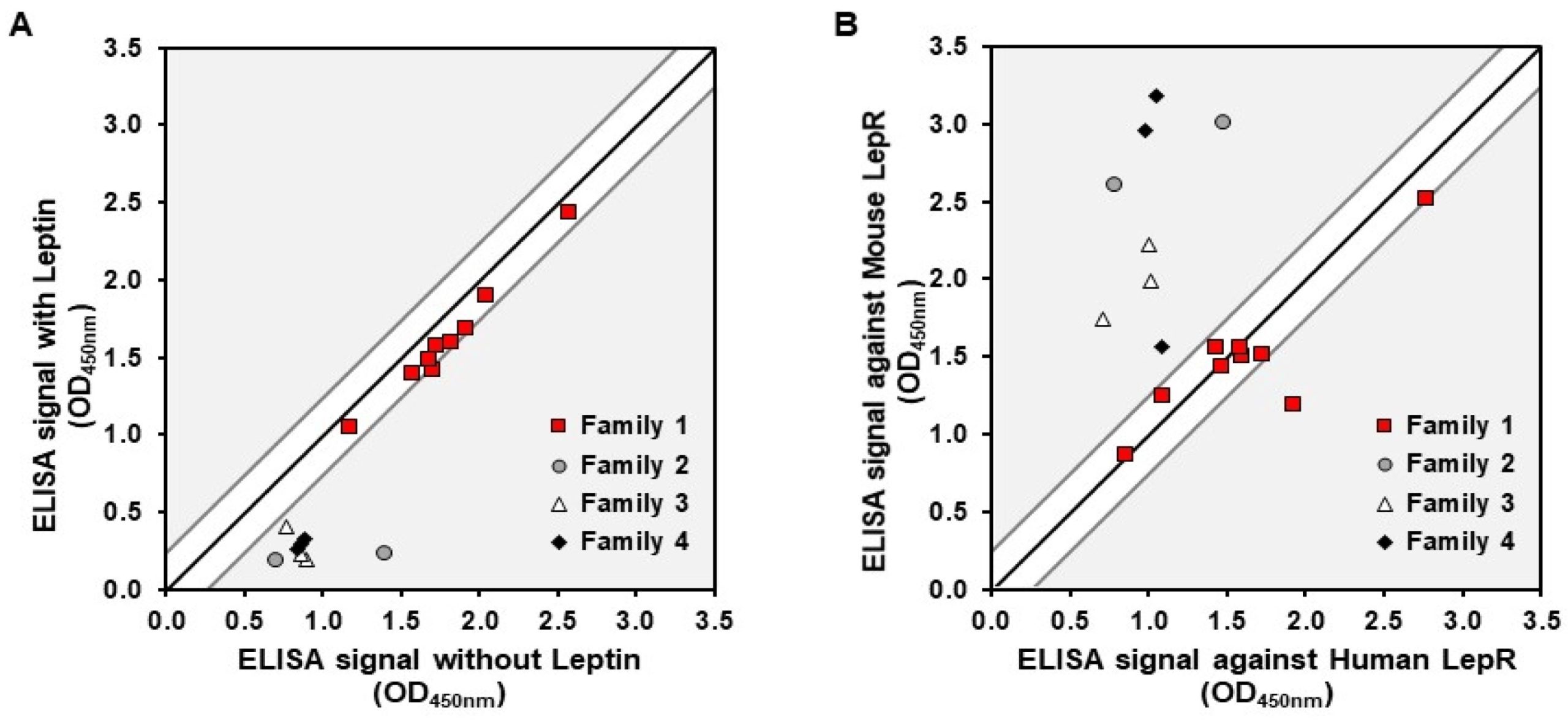
In this proof-of-concept study, we investigate the potential of employing a delivery strategy based on anti-LepR Nanofitins as molecular shuttles to enhance the transport of biologics through the intestine. In vitro, our approach involved developing Nanofitins targeting the LepR. The target product profile included non-competition with the leptin binding to its receptor, aimed at improving safety by minimizing competition with endogenous ligands, and cross-reactivity with receptors from human, mouse, and pig hosts to suit relevant ex vivo and in vivo preclinical models. Ex vivo, we successfully demonstrated the potential of an anti-LepR Nanofitin candidate as an efficient carrier for transporting functional cargo proteins across the intestinal barrier, suggesting the potential of the NF scaffold for application in oral biologics delivery strategies.
Download the full article as PDF here: Enhancing Oral Delivery of Biologics
or read it here
Masloh, S.; Chevrel, A.; Culot, M.; Perrocheau, A.; Kalia, Y.N.; Frehel, S.; Gaussin, R.; Gosselet, F.; Huet, S.; Zeisser Labouebe, M.; et al. Enhancing Oral Delivery of Biologics: A Non-Competitive and Cross-Reactive Anti-Leptin Receptor Nanofitin Demonstrates a Gut-Crossing Capacity in an Ex Vivo Porcine Intestinal Model. Pharmaceutics 2024, 16, 116. https://doi.org/10.3390/pharmaceutics16010116
Read more on Orally Disintegrating Tablets (ODTs) here:


