Formulation, characterization, pharmacokinetics and antioxidant activity of phloretin oral granules
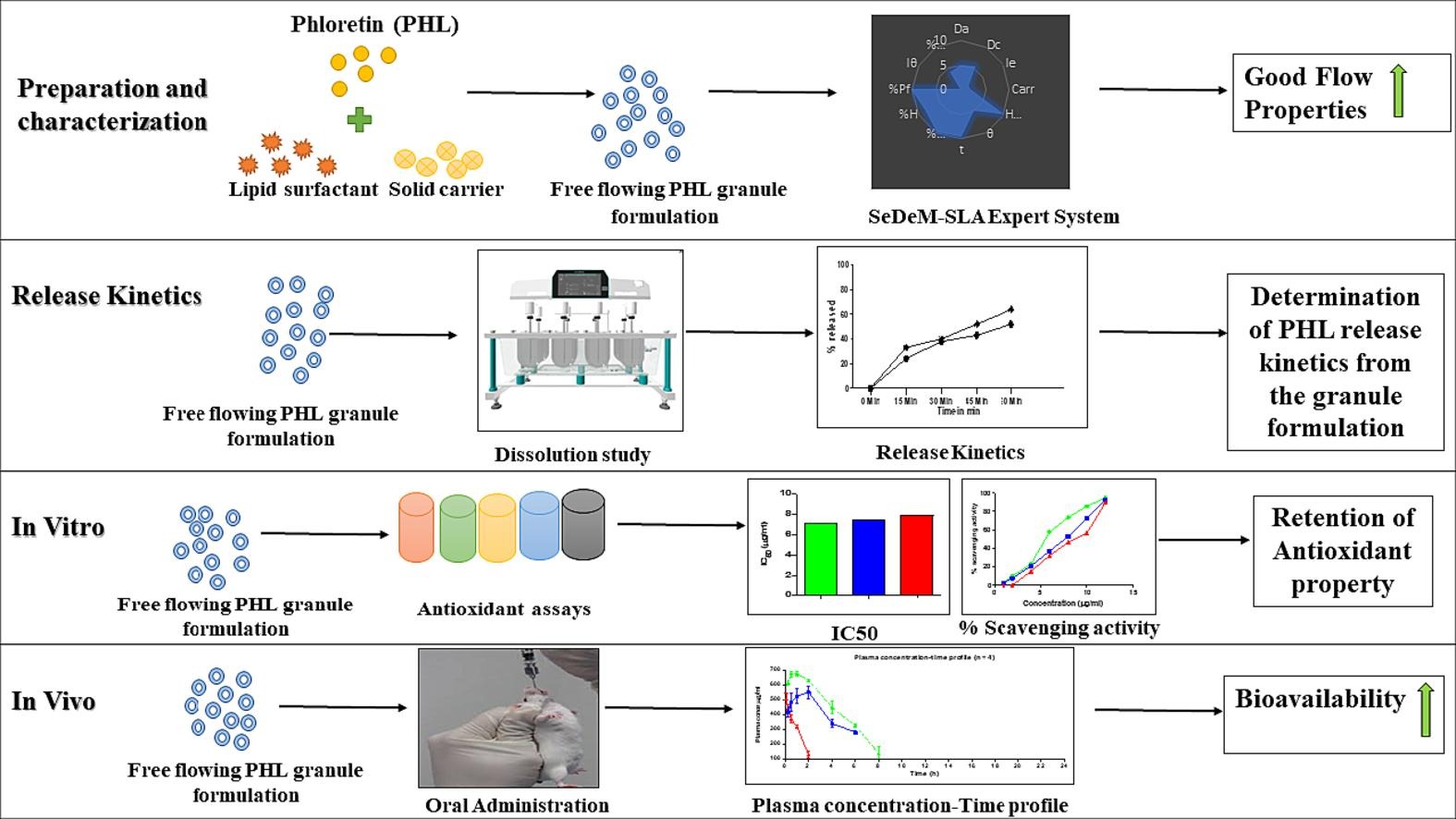
Phloretin (PHL), a flavonoid of the dihydrogen chalcone class, is reported to have low oral bioavailability due to its poor solubility and absorption. A common approach to enhance the solubility of such flavonoids is solubilization in a polymeric or lipidic matrix which would help in enhance dissolution rate and solubility. Accordingly, in the current study PHL was dissolved in Gelucire® 44/14 by melt-fusion technique and the viscous semisolid melt was adsorbed on a solid carrier to obtain free flowing granules. SeDeM-SLA (Solid-Liquid Adsorption) expert system was employed to select the most suitable carrier. This study achieved positive outcomes through the successful development of formulated oral PHL granules. The granules exhibited good stability, and favourable pharmacokinetic properties. In addition, the selected carrier effectively retained the antioxidant properties of PHL.
Introduction
Flavonoids are biologically active molecules found in many herbal food sources and have various health benefits (Roy et al., 2022a). Their efficacy in reducing the risk of several diseases has led to increasing interest in consuming a diet rich in flavonoids such as fruits and vegetables (Wu et al., 2019). One such flavonoid is Phloretin (PHL), a dihydrogen chalcone found naturally in significant quantities in apples and strawberries. PHL is available in a visually appealing pearl white powder. It has a melting point of 263.5 °C, signifying its stability under high-temperature conditions. Regarding its solubility, PHL displays a limited ability to dissolve in water, and it only shows modest solubility in other common solvents such as methanol, ethanol, and DMSO. This property can be advantageous in certain applications where controlled dissolution is desired (Nakhate et al., 2022). PHL is classified as a Biopharmaceutics Classification System (BCS) class II drug, which means it has low solubility and high permeability (Lu et al., 2023).
This classification suggests that the absorption of PHL in the body may be limited, potentially hampering its effectiveness as a therapeutic agent. PHL possesses anti-inflammatory properties that have been demonstrated to reduce inflammation in various chronic illnesses (Huang et al., 2015a, Wei et al., 2017). Additionally, it displays potent antioxidant characteristics, enabling it to counteract destructive free radicals within the body, thereby diminishing oxidative stress and cell damage (Wei et al., 2017). Studies have also revealed its anti-cancer properties, as it can impede the growth of cancerous cells (Chen et al., 2021, Choi, 2019, Kim et al., 2022, Roy et al., 2022b, Yang et al., 2020). Furthermore, PHL exhibits antidiabetic properties and may aid in regulating blood sugar levels in individuals with diabetes (Habtemariam, 2023, Mariadoss et al., 2019). Due to its antioxidant properties, PHL has been shown to protect the skin against damage caused by UV radiation (Casarini et al., 2020, Shin et al., 2014). As per an in-house study conducted, it has been established that PHL exhibits nootropic, neuroprotective, and neurotrophic properties (Ghumatkar et al., 2018, Ghumatkar et al., 2015, Ghumatkar et al., 2019).
Although PHL has ample therapeutic effects, its pharmacokinetic (PK) properties are not optimal because it has low bioavailability and is rapidly and extensively metabolized. The oral bioavailability of a drug is influenced by several factors such as aqueous solubility, drug permeability, dissolution rate, first-pass metabolism, and susceptibility to efflux mechanisms. Efflux transporters play a key role in regulating the absorption, distribution, metabolism, and excretion (ADME) of many drugs and endogenous compounds in the body. It should be noted that aqueous solubility and drug permeability are two critical parameters that significantly impact the oral bioavailability of a drug. Recent studies have reported that PHL is a substrate of P-glycoprotein transporter. The pharmacokinetics of PHL can be influenced by the inhibitors or inducers of these transporters, leading to either increased or decreased absorption and bioavailability (Zhao et al., 2020). Despite exhibiting strong pharmacological activity in vitro, the effectiveness of PHL is limited owing to its low oral bioavailability in vivo which restricts its use as a therapeutic agent. However, further research is required to develop an oral formulation of PHL that exhibits good bioavailability, biodistribution, and efficacy. There are many solubility improvement techniques available which can be categorized into physical modifications, chemical modifications, and miscellaneous methods.
Physical modifications include particle size reduction (micronization, nanosuspension), crystal habit modification (polymorphs, amorphous form, cocrystallization), and drug dispersion in carriers (eutectic mixtures, solid dispersions, solid solutions, cryogenic techniques). Chemical modifications involve pH change, use of buffers, derivatization, complexation, and salt formation. Miscellaneous methods include supercritical fluid process, use of adjuvants (surfactants, solubilizers, cosolvency, hydrotrophy), and novel lipid excipients (Moravkar et al., 2017, Savjani et al., 2012). Scientists have been working on enhancing the solubility of PHL using steviol glycoside (STE)-based micelles (MC) and solid dispersions (SD) (F. Wang et al., 2020). Another study aimed to improve the oral bioavailability and bioefficacy of PHL by exploring mixed polymers Pluronic® F127 and P123, which modified various triglycerides such as MAISINE® 35–1 (LCT), medium-chain triglycerides (MCT), and glyceryl tributyrate (SCT) in self-nanoemulsions (SNEs) (Wang et al., 2020a, Wang et al., 2020b).
Additionally, combining PHL with cyclodextrin resulted in improved water solubility, forming phloretin–methyl-β-cyclodextrin (phloretin–Me-β-CD) and phloretin–(2-hydroxy)propyl-β-cyclodextrin (phloretin–HP-β-CD) through the freeze-drying method (Hu, Zhou, Han, Li, & Zhou, 2020). The present study aimed to develop an oral formulation of PHL with enhanced bioavailability with a well-known formulation strategy, which involves the use of lipidic surfactants used to dissolve the active (Kanikkannan, 2018, Mu et al., 2013, van der Merwe et al., 2020). This form of the active compound shows a higher solubility, and dissolution rate and therefore, better bioavailability. One of the most frequently used lipidic surfactants is Gelucire® 44/14, which assists in the dissolution of active substances that are poorly soluble. Gelucire® 44/14 is a commonly used for the oral delivery of poorly water-soluble drugs. It belongs to the class of self-emulsifying excipients, which are designed to enhance the solubility and bioavailability of hydrophobic drugs. Gelucire® 44/14 is a mixture of mono-, di-, and triglycerides of fatty acids, polyethylene glycol (PEG) esters of fatty acids, and free polyethylene glycol. It has a unique composition that allows it to form a stable, semi-solid self-emulsifying system when dispersed in an aqueous medium.
When a poorly water-soluble drug is combined with Gelucire ®44/14, the excipient forms a self-emulsifying system upon contact with gastrointestinal fluids. This self-emulsification process leads to the formation of fine oil-in-water emulsion droplets, increasing the surface area of the drug and promoting its dissolution. As a result, the drug’s absorption and bioavailability are improved. Gelucire® 44/14 offers several advantages as an excipient, including its ability to enhance drug solubility, its stability in various environmental conditions, and its compatibility with different drug compounds (Aungst et al., 1997, Chambin and Jannin, 2005, da Fonseca Antunes et al., 2013, Devireddy and Veerareddy, 2012, Fernandez et al., 2008, Jia et al., 2023, Parmar et al., 2012, Shaker, 2018, Tanaka et al., 2012, Yüksel et al., 2003). Gelucire® 44/14 may have potential to enhance the bioavailability of PHL by inhibiting its efflux through Pg-p since PHL is a substrate of Pg-p (Bansal et al., 2009, Husain et al., 2022, Jia et al., 2023, Mai et al., 2022, Pinjari et al., 2017, Sachs-Barrable et al., 2008, Sachs-Barrable et al., 2007).
When an active substance is processed with Gelucire® 44/14, a semisolid viscous paste is formed owing to the waxy nature of the lipid. This semisolid paste can be adsorbed onto high surface area solid carriers to produce a free-flowing powder. There are different types of solid carriers available that can be used for adsorption due to their high surface area. These carriers, like silica, come in various forms and have different properties such as surface area, particle and pore size, and structure (amorphous or crystalline). These properties can affect how much drug/ liquid can be loaded onto the carrier and how it is released. Silica can also be used to achieve sustained or targeted drug release and protect the drugs from premature release and degradation.
In this study, two specific carriers, Neusilin® US2 and Syloid 244 FP, were chosen to evaluate their potential for developing an effective oral formulation. These carriers have been shown to be better than others in previous research studies (Alwadei et al., 2019, Jadhav et al., 2017, Kovačević et al., 2022). The carrier which gave a free-flowing powder with good flow properties at a lower carrier to liquid formulation ratio was selected for further studies. The optimization for selection of the solid carrier was done using the SeDeM-SLA expert system which was recently proposed for selecting the most suitable solid carrier for adsorption (Shah et al., 2021). The first formulation (F1) comprised of PHL, Gelucire® 44/14 and Neusilin® US2. Neusilin® US2 is amorphous magnesium aluminosilicate used as drug solid carrier (Kostelanská et al., 2022). It has the capacity to adsorb large amounts of oils or other liquids and can be mechanically compacted into superior quality of powders, granules, and tablets (Dangre et al., 2022, Jha et al., 2020, Mura et al., 2019). Neusilin® US2 forms smooth granules and retains the drugs in their stable amorphous form. It can also help to improve the bioavailability and stability of poorly soluble drugs (Dangre et al., 2022). The second formulation (F2) contained, PHL, Gelucire® 44/14 and Syloid 244 FP. Syloid 244 FP is a synthetic silica that is a commonly used excipient in the pharmaceutical industry as an adsorbent. It is known for its high surface area, uniform particle size distribution, and porous structure, which makes it an effective adsorbent and carrier for active pharmaceutical ingredients (Bhattacharyya and Ramachandran, 2022, Kumar et al., 2018). Syloid 244 FP is often used to improve the flowability and compressibility of powders as well as to enhance the stability of active pharmaceutical excipients (APIs) by protecting them from moisture and oxidation. The quantity of pharmaceutical excipients used in both formulations were selected as per the Generally Recognized as Safe (GRAS) regulations and limitations.
Furthermore, the optimized formulation was evaluated for in vitro PHL release in simulated gastric fluid (SGF) and intestinal fluids (SIF). PHL has a well-proven strong antioxidant activity; however, it is important to analyze the formulations for retention of the same efficacy. Formulation strategies can affect the stability and bioavailability of PHL, which in turn can affect its antioxidant activity. The formulations also contain ingredients that can interact and affect its activity. Therefore, it is important to test the antioxidant activity of the formulation to ensure that the antioxidant is still effective and can provide the desired benefits (Atun et al., 2020, Sousa et al., 2021). The individual antioxidant properties of the excipients chosen in the present study have not been reported yet. Therefore, the presence of these excipients is unlikely to interfere with the antioxidant assays.
To assess the antioxidant properties of the PHL formulations following assays were conducted; DPPH (1,1-diphenyl-2-picryl hydrazyl) Radical Scavenging Assay, Iron chelation, Nitric Oxide (NO) Radical Scavenging Activity, ABTS (2,2′-azinobis-[3-ethylbenzthiazoline-6-sulphonic acid]) assay, Ferric reducing antioxidant power (FRAP) assay, and Oxygen Radical Absorbance Capacity (ORAC). These assays differ in their sensitivity, specificity, and reproducibility, and their results can be affected by various factors, such as pH, temperature, and solvent (Du et al., 2009, Dudonne et al., 2009, Thaipong et al., 2006). Therefore, it is important to use multiple assays to obtain a comprehensive assessment of the antioxidant potential of a substance and to account for the limitations and variability of each assay. These assays were crucial in confirming that the PHL could be released from its granular formulation and exhibit antioxidant properties. Additionally, an in vivo pharmacokinetic study was performed to understand the bioavailability of pure PHL versus F1. This is a first of its kind study to develop oral PHL granules using a lipidic surfactant and to examine their physicochemical properties, in vitro assays, and in vivo pharmacokinetics.
Read more
Radni D. Deshpande, Devanshi S. Shah, Sharda Gurram, Durgesh K. Jha, Paramita Batabyal, Purnima D. Amin, Sadhana Sathaye, Formulation, characterization, pharmacokinetics and antioxidant activity of phloretin oral granules, International Journal of Pharmaceutics, Volume 645, 2023, 123386, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123386.

