Improved pharmacokinetic and lymphatic uptake of Rose Bengal after transfersome intradermal deposition using hollow microneedles
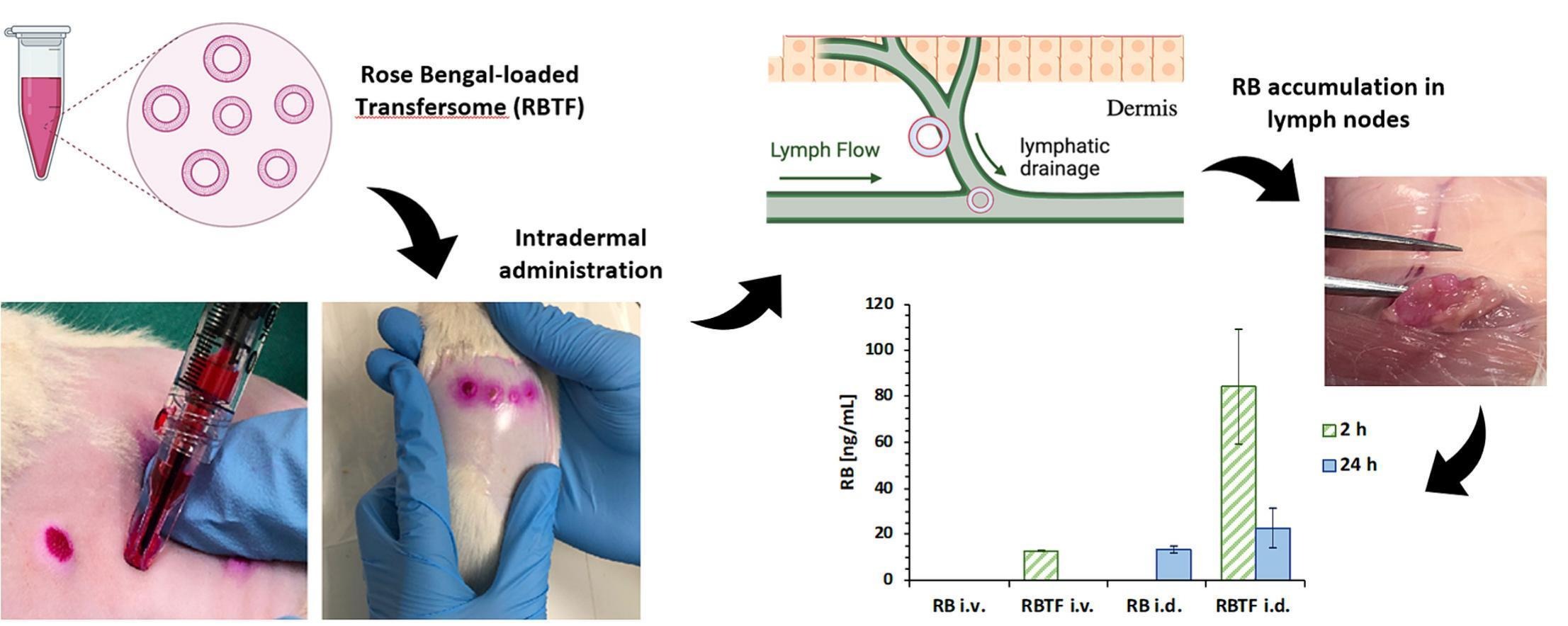
The lymphatic system is active in several processes that regulate human diseases, among which cancer progression stands out. Thus, various drug delivery systems have been investigated to promote lymphatic drug targeting for cancer therapy; mainly, nanosized particles in the 10–150 nm range quickly achieve lymphatic vessels after an interstitial administration. Herein, a strategy to boost the lymphotropic delivery of Rose Bengal (RB), a hydrosoluble chemotherapeutic, is proposed, and it is based on the loading into Transfersomes (RBTF) and their intradermal deposition in vivo by microneedles. RBTF of 96.27 ± 13.96 nm (PDI = 0.29 ± 0.02) were prepared by a green reverse-phase evaporation technique, and they showed an RB encapsulation efficiency of 98.54 ± 0.09%.
In vitro, RBTF remained physically stable under physiological conditions and avoided the release of RB. In vivo, intravenous injection of RBTF prolonged RB half-life of 50 min in healthy rats compared to RB intravenous injection; the RB half-life in rat body was further increased after intradermal injection reaching 24 h, regardless of the formulation used. Regarding lymphatic targeting, RBTF administered intravenously provided an RB accumulation in the lymph nodes of 12.3 ± 0.14 ng/mL after 2 h, whereas no RB accumulation was observed after RB intravenous injection.
Highlights
- RBTF are physically stable in physiological states and prevent premature RB release.
- RBTF increase RB half-life from 30 to 90 min when intravenously injected.
- RB half-life increases up to 24 h after intradermal injection of RBTF and RB solution.
- RBTF highly potentiate RB lymph-node accumulation when intradermally deposited.
- RBTF circulation in the lymphatics encourages their evaluation for cancer therapy.
Intradermally administered RBTF resulted in the highest RB amount detected in lymph nodes after 2 h from the injection (84.2 ± 25.10 ng/mL), which was even visible to the naked eye based on the pink colouration of the drug. In the case of intradermally administered RB, RB in lymph node was detected only at 24 h (13.3 ± 1.41 ng/mL). In conclusion, RBTF proved an efficient carrier for RB delivery, enhancing its pharmacokinetics and promoting lymph-targeted delivery. Thus, RBTF represents a promising nanomedicine product for potentially facing the medical need for novel strategies for cancer therapy.
Download the full article as PDF here Improved pharmacokinetic and lymphatic uptake of Rose Bengal after transfersome intradermal deposition using hollow microneedles
Read more here
Materials
Rose Bengal disodium salt (RB), cholesterol, Span® 80, HPLC grade methanol, ammonium acetate, sodium hydroxide, phosphate-buffered saline (PBS) tablets (pH 7.3–7.5), dimethyl sulfoxide (DMSO) were purchased by Sigma-Aldrich (St. Louis, MO). Lipoid S100® was a kind gift from Lipoid GmbH (Ludwigshafen, Germany). Uranyl acetate (gadolinium acetate tetrahydrate) was purchased by Ted Pella Inc. (Redding, CA). Ethanol (industrial methylated spirit 99.5%) was obtained by Atomic Scientific (Manchester, UK). Ultrapure water was obtained from a water purification system (Elga PURELAB DV 25, Veolia Water Systems, Dublin, Ireland). Horse Serum was of UK origin and was obtained by Thermo Scientific (Hampshire, England). Nanosoft® HMNs were purchased from Fillmed (Paris, France).
Sara Demartis, Giovanna Rassu, Qonita Kurnia Anjani, Fabiana Volpe-Zanutto, Aaron R.J. Hutton, Akmal B. Sabri, Helen O. McCarthy, Paolo Giunchedi, Ryan F. Donnelly, Elisabetta Gavini, Improved pharmacokinetic and lymphatic uptake of Rose Bengal after transfersome intradermal deposition using hollow microneedles, Journal of Controlled Release, Volume 369, 2024, Pages 363-375, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2024.03.048.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


